Cardiac MyBP-C regulates the rate and force of contraction in mammalian myocardium
- PMID: 25552695
- PMCID: PMC4283578
- DOI: 10.1161/CIRCRESAHA.116.300561
Cardiac MyBP-C regulates the rate and force of contraction in mammalian myocardium
Abstract
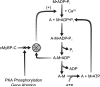
Cardiac myosin-binding protein-C (cMyBP-C) is a thick filament-associated protein that seems to contribute to the regulation of cardiac contraction through interactions with either myosin or actin or both. Several studies over the past several years have suggested that the interactions of cardiac myosin-binding protein-C with its binding partners vary with its phosphorylation state, binding predominantly to myosin when dephosphorylated and to actin when it is phosphorylated by protein kinase A or other kinases. Here, we summarize evidence suggesting that phosphorylation of cardiac myosin binding protein-C is a key regulator of the kinetics and amplitude of cardiac contraction during β-adrenergic stimulation and increased stimulus frequency. We propose a model for these effects via a phosphorylation-dependent regulation of the kinetics and extent of cooperative recruitment of cross bridges to the thin filament: phosphorylation of cardiac myosin binding protein-C accelerates cross bridge binding to actin, thereby accelerating recruitment and increasing the amplitude of the cardiac twitch. In contrast, enhanced lusitropy as a result of phosphorylation seems to be caused by a direct effect of phosphorylation to accelerate cross-bridge detachment rate. Depression or elimination of one or both of these processes in a disease, such as end-stage heart failure, seems to contribute to the systolic and diastolic dysfunction that characterizes the disease.
Keywords: cardiac myosin binding protein-C; phosphorylation; regulation.
© 2015 American Heart Association, Inc.
Figures







References
-
- Offer G, Moos C, Starr R. A new protein of the thick filaments of vertebrate skeletal myofibrils. Extractions, purification and and characterization. J Mol Biol. 1973;74:653–676. - PubMed
-
- Moos C, Mason CM, Besterman JM, Feng IN, Dubin JH. The binding of skeletal muscle C-protein to F-actin, and its relation to the interaction of actin with myosin subfragment-1. J Mol Biol. 1978;124:571–586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

