Ebola virus and severe acute respiratory syndrome coronavirus display late cell entry kinetics: evidence that transport to NPC1+ endolysosomes is a rate-defining step
- PMID: 25552710
- PMCID: PMC4325712
- DOI: 10.1128/JVI.03398-14
Ebola virus and severe acute respiratory syndrome coronavirus display late cell entry kinetics: evidence that transport to NPC1+ endolysosomes is a rate-defining step
Abstract
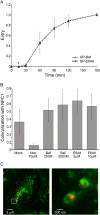
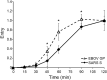
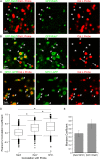
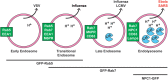
Ebola virus (EBOV) causes hemorrhagic fevers with high mortality rates. During cellular entry, the virus is internalized by macropinocytosis and trafficked through endosomes until fusion between the viral and an endosomal membrane is triggered, releasing the RNA genome into the cytoplasm. We found that while macropinocytotic uptake of filamentous EBOV viruslike particles (VLPs) expressing the EBOV glycoprotein (GP) occurs relatively quickly, VLPs only begin to enter the cytoplasm after a 30-min lag, considerably later than particles bearing the influenza hemagglutinin or GP from lymphocytic choriomeningitis virus, which enter through late endosomes (LE). For EBOV, the long lag is not due to the large size or unusual shape of EBOV filaments, the need to prime EBOV GP to the 19-kDa receptor-binding species, or a need for unusually low endosomal pH. In contrast, since we observed that EBOV entry occurs upon arrival in Niemann-Pick C1 (NPC1)-positive endolysosomes (LE/Lys), we propose that trafficking to LE/Lys is a key rate-defining step. Additional experiments revealed, unexpectedly, that severe acute respiratory syndrome (SARS) S-mediated entry also begins only after a 30-min lag. Furthermore, although SARS does not require NPC1 for entry, SARS entry also begins after colocalization with NPC1. Since the only endosomal requirement for SARS entry is cathepsin L activity, we tested and provide evidence that NPC1(+) LE/Lys have higher cathepsin L activity than LE, with no detectable activity in earlier endosomes. Our findings suggest that both EBOV and SARS traffic deep into the endocytic pathway for entry and that they do so to access higher cathepsin activity.
Importance: Ebola virus is a hemorrhagic fever virus that causes high fatality rates when it spreads from zoonotic vectors into the human population. Infection by severe acute respiratory syndrome coronavirus (SARS-CoV) causes severe respiratory distress in infected patients. A devastating outbreak of EBOV occurred in West Africa in 2014, and there was a significant outbreak of SARS in 2003. No effective vaccine or treatment has yet been approved for either virus. We present evidence that both viruses traffic late into the endocytic pathway, to NPC1(+) LE/Lys, in order to enter host cells, and that they do so to access high levels of cathepsin activity, which both viruses use in their fusion-triggering mechanisms. This unexpected similarity suggests an unexplored vulnerability, trafficking to NPC1(+) LE/Lys, as a therapeutic target for SARS and EBOV.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
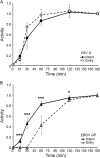

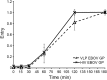
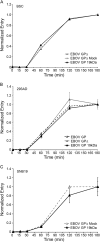

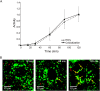

References
-
- Gire SK, Goba A, Andersen KG, Sealfon RSG, Park DJ, Kanneh L, Jalloh S, Momoh M, Fullah M, Dudas G, Wohl S, Moses LM, Yozwiak NL, Winnicki S, Matranga CB, Malboeuf CM, Qu J, Gladden AD, Schaffner SF, Yang X, Jiang PP, Nekoui M, Colubri A, Coomber MR, Fonnie M, Moigboi A, Gbakie M, Kamara FK, Tucker V, Konuwa E, Saffa S, Sellu J, Jalloh AA, Kovoma A, Koninga J, Mustapha I, Kargbo K, Foday M, Yillah M, Kanneh F, Robert W, Massally JLB, Chapman SB, Bochicchio J, Murphy C, Nusbaum C, Young S, Birren BW, Grant DS, Scheiffelin JS, Lander ES, Happi C, Gevao SM, Gnirke A, Rambaut A, Garry RF, Khan SH, Sabeti PC. 2014. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 345:1369–1372. doi:10.1126/science.1259657. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

