Transcriptome analysis of Kaposi's sarcoma-associated herpesvirus during de novo primary infection of human B and endothelial cells
- PMID: 25552714
- PMCID: PMC4337554
- DOI: 10.1128/JVI.02507-14
Transcriptome analysis of Kaposi's sarcoma-associated herpesvirus during de novo primary infection of human B and endothelial cells
Abstract
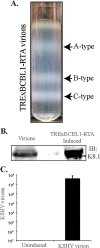
Kaposi's sarcoma-associated herpesvirus (KSHV) infects many target cells (e.g., endothelial, epithelial, and B cells, keratinocytes, and monocytes) to establish lifelong latent infections. Viral latent-protein expression is critical in inducing and maintaining KSHV latency. Infected cells are programmed to retain the incoming viral genomes during primary infection. Immediately after infection, KSHV transcribes many lytic genes that modulate various cellular pathways to establish successful infection. Analysis of the virion particle showed that the virions contain viral mRNAs, microRNAs, and other noncoding RNAs that are transduced into the target cells during infection, but their biological functions are largely unknown. We performed a comprehensive analysis of the KSHV virion packaged transcripts and the profiles of viral genes transcribed after de novo infections of various cell types (human peripheral blood mononuclear cells [PBMCs], CD14(+) monocytes, and telomerase-immortalized vascular endothelial [TIVE] cells), from viral entry until latency establishment. A next-generation sequence analysis of the total transcriptome showed that several viral RNAs (polyadenylated nuclear RNA, open reading frame 58 [ORF58], ORF59, T0.7, and ORF17) were abundantly present in the KSHV virions and effectively transduced into the target cells. Analysis of the transcription profiles of each viral gene showed specific expression patterns in different cell lines, with the majority of the genes, other than latent genes, silencing after 24 h postinfection. We differentiated the actively transcribing genes from the virion-transduced transcripts using a nascent RNA capture approach (Click-iT chemistry), which identified transcription of a number of viral genes during primary infection. Treating the infected cells with phosphonoacetic acid (PAA) to block the activity of viral DNA polymerase confirmed the involvement of lytic DNA replication during primary infection. To further understand the role of DNA replication during primary infection, we performed de novo PBMC infections with a recombinant ORF59-deleted KSHV virus, which showed significantly reduced numbers of viral copies in the latently infected cells. In summary, the transduced KSHV RNAs as well as the actively transcribed genes control critical processes of early infection to establish KSHV latency.
Importance: Kaposi's sarcoma-associated herpesvirus (KSHV) is the causative agent of multiple human malignancies in immunocompromised individuals. KSHV establishes a lifelong latency in the infected host, during which only a limited number of viral genes are expressed. However, a fraction of latently infected cells undergo spontaneous reactivation to produce virions that infect the surrounding cells. These newly infected cells are primed early to retain the incoming viral genome and induce cell growth. KSHV transcribes a variety of lytic proteins during de novo infections that modulate various cellular pathways to establish the latent infection. Interestingly, a large number of viral proteins and RNA are encapsidated in the infectious virions and transduced into the infected cells during a de novo infection. This study determined the kinetics of the viral gene expression during de novo KSHV infections and the functional role of the incoming viral transcripts in establishing latency.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures








Similar articles
-
Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression.J Virol. 2004 Apr;78(7):3601-20. doi: 10.1128/jvi.78.7.3601-3620.2004. J Virol. 2004. PMID: 15016882 Free PMC article.
-
Kaposi's Sarcoma-Associated Herpesvirus Infection Induces the Expression of Neuroendocrine Genes in Endothelial Cells.J Virol. 2020 Mar 31;94(8):e01692-19. doi: 10.1128/JVI.01692-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31969437 Free PMC article.
-
Spindle cell conversion by Kaposi's sarcoma-associated herpesvirus: formation of colonies and plaques with mixed lytic and latent gene expression in infected primary dermal microvascular endothelial cell cultures.J Virol. 2001 Jun;75(12):5614-26. doi: 10.1128/JVI.75.12.5614-5626.2001. J Virol. 2001. PMID: 11356969 Free PMC article.
-
KSHV LANA--the master regulator of KSHV latency.Viruses. 2014 Dec 11;6(12):4961-98. doi: 10.3390/v6124961. Viruses. 2014. PMID: 25514370 Free PMC article. Review.
-
Pathological Features of Kaposi's Sarcoma-Associated Herpesvirus Infection.Adv Exp Med Biol. 2018;1045:357-376. doi: 10.1007/978-981-10-7230-7_16. Adv Exp Med Biol. 2018. PMID: 29896675 Review.
Cited by
-
Kaposi's sarcoma-associated herpesvirus vIRF2 protein utilizes an IFN-dependent pathway to regulate viral early gene expression.PLoS Pathog. 2019 May 6;15(5):e1007743. doi: 10.1371/journal.ppat.1007743. eCollection 2019 May. PLoS Pathog. 2019. PMID: 31059555 Free PMC article.
-
Constitutive Activation of Interleukin-13/STAT6 Contributes to Kaposi's Sarcoma-Associated Herpesvirus-Related Primary Effusion Lymphoma Cell Proliferation and Survival.J Virol. 2015 Oct;89(20):10416-26. doi: 10.1128/JVI.01525-15. Epub 2015 Aug 5. J Virol. 2015. PMID: 26246572 Free PMC article.
-
KSHV 3.0: a state-of-the-art annotation of the Kaposi's sarcoma-associated herpesvirus transcriptome using cross-platform sequencing.mSystems. 2024 Feb 20;9(2):e0100723. doi: 10.1128/msystems.01007-23. Epub 2024 Jan 11. mSystems. 2024. PMID: 38206015 Free PMC article.
-
Primaquine as a Candidate for HHV-8-Associated Primary Effusion Lymphoma and Kaposi's Sarcoma Treatment.Cancers (Basel). 2022 Jan 21;14(3):543. doi: 10.3390/cancers14030543. Cancers (Basel). 2022. PMID: 35158811 Free PMC article.
-
A Panel of Kaposi's Sarcoma-Associated Herpesvirus Mutants in the Polycistronic Kaposin Locus for Precise Analysis of Individual Protein Products.J Virol. 2022 Mar 9;96(5):e0156021. doi: 10.1128/JVI.01560-21. Epub 2021 Dec 22. J Virol. 2022. PMID: 34936820 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

