Differential effects of the G118R, H51Y, and E138K resistance substitutions in different subtypes of HIV integrase
- PMID: 25552724
- PMCID: PMC4337543
- DOI: 10.1128/JVI.03353-14
Differential effects of the G118R, H51Y, and E138K resistance substitutions in different subtypes of HIV integrase
Abstract
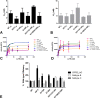
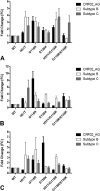
Dolutegravir (DTG) is the latest antiretroviral (ARV) approved for the treatment of human immunodeficiency virus (HIV) infection. The G118R substitution, previously identified with MK-2048 and raltegravir, may represent the initial substitution in a dolutegravir resistance pathway. We have found that subtype C integrase proteins have a low enzymatic cost associated with the G118R substitution, mostly at the strand transfer step of integration, compared to either subtype B or recombinant CRF02_AG proteins. Subtype B and circulating recombinant form AG (CRF02_AG) clonal viruses encoding G118R-bearing integrases were severely restricted in their viral replication capacity, and G118R/E138K-bearing viruses had various levels of resistance to dolutegravir, raltegravir, and elvitegravir. In cell-free experiments, the impacts of the H51Y and E138K substitutions on resistance and enzyme efficiency, when present with G118R, were highly dependent on viral subtype. Sequence alignment and homology modeling showed that the subtype-specific effects of these mutations were likely due to differential amino acid residue networks in the different integrase proteins, caused by polymorphic residues, which significantly affect native protein activity, structure, or function and are important for drug-mediated inhibition of enzyme activity. This preemptive study will aid in the interpretation of resistance patterns in dolutegravir-treated patients.
Importance: Recognized drug resistance mutations have never been reported for naive patients treated with dolutegravir. Additionally, in integrase inhibitor-experienced patients, only R263K and other previously known integrase resistance substitutions have been reported. Here we suggest that alternate resistance pathways may develop in non-B HIV-1 subtypes and explain how "minor" polymorphisms and substitutions in HIV integrase that are associated with these subtypes can influence resistance against dolutegravir. This work also highlights the importance of phenotyping versus genotyping when a strong inhibitor such as dolutegravir is being used. By characterizing the G118R substitution, this work also preemptively defines parameters for a potentially important pathway in some non-B HIV subtype viruses treated with dolutegravir and will aid in the inhibition of such a virus, if detected. The general inability of strand transfer-related substitutions to diminish 3' processing indicates the importance of the 3' processing step and highlights a therapeutic angle that needs to be better exploited.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





References
-
- Kommer C. 2013. New UNAIDS figures. Kinderkrankenschwester 32:78 (In German.). - PubMed
-
- International Association of Physicians in AIDS Care. 2003. HAART slashed AIDS death rates by 80 percent. IAPAC Mon 9:279. - PubMed
-
- Williams A, Friedland G. 1997. Adherence, compliance, and HAART. AIDS Clin Care 9:51–54, 58. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

