Astrocytes protect neurons from Aβ1-42 peptide-induced neurotoxicity increasing TFAM and PGC-1 and decreasing PPAR-γ and SIRT-1
- PMID: 25552918
- PMCID: PMC4278875
- DOI: 10.7150/ijms.10035
Astrocytes protect neurons from Aβ1-42 peptide-induced neurotoxicity increasing TFAM and PGC-1 and decreasing PPAR-γ and SIRT-1
Abstract
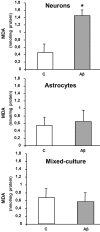
One of the earliest neuropathological events in Alzheimer's disease is accumulation of astrocytes at sites of Aβ1-42 depositions. Our results indicate that Aβ1-42 toxic peptide increases lipid peroxidation, apoptosis and cell death in neurons but not in astrocytes in primary culture. Aβ1-42-induced deleterious neuronal effects are not present when neurons and astrocytes are mixed cultured. Stimulation of astrocytes with toxic Aβ1-42 peptide increased p-65 and decreased IκB resulting in inflammatory process. In astrocytes Aβ1-42 decreases protein expressions of sirtuin 1 (SIRT-1) and peroxisome proliferator-activated receptor γ (PPAR-γ) and over-expresses peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1) and mitochondrial transcription factor A (TFAM), protecting mitochondria against Aβ1-42-induced damage and promoting mitochondrial biogenesis. In summary our data suggest that astrocytes may have a key role in protecting neurons, increasing neural viability and mitochondrial biogenesis, acquiring better oxidative stress protection and perhaps modulating inflammatory processes against Aβ1-42 toxic peptide. This might be a sign of a complex epigenetic process in Alzheimer's disease development.
Keywords: Alzheimer's Disease; MnSOD; NF-κB.; PGC-1; PPAR-γ; TFAM.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures






References
-
- Hardy JA, Higgins GA. Alzheimer´s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. - PubMed
-
- Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
-
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. - PubMed
-
- Trushina E, McMurray CT. Oxidative stress and mitochondrial dysfunction in neurodegenerative diseases. Neuroscience. 2007;145:1233–1248. - PubMed
-
- Vallés SL, Borrás C, Furriol J. et al. Estradiol or genistein rescues neurons from Abeta-induced cell death by inhibiting activation of p38. Aging Cell. 2008;7:112–118. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

