Macrophage targeted theranostics as personalized nanomedicine strategies for inflammatory diseases
- PMID: 25553105
- PMCID: PMC4279001
- DOI: 10.7150/thno.9476
Macrophage targeted theranostics as personalized nanomedicine strategies for inflammatory diseases
Abstract
Inflammatory disease management poses challenges due to the complexity of inflammation and inherent patient variability, thereby necessitating patient-specific therapeutic interventions. Theranostics, which integrate therapeutic and imaging functionalities, can be used for simultaneous imaging and treatment of inflammatory diseases. Theranostics could facilitate assessment of safety, toxicity and real-time therapeutic efficacy leading to personalized treatment strategies. Macrophages are an important cellular component of inflammatory diseases, participating in varied roles of disease exacerbation and resolution. The inherent phagocytic nature, abundance and disease homing properties of macrophages can be targeted for imaging and therapeutic purposes. This review discusses the utility of theranostics in macrophage ablation, phenotype modulation and inhibition of their inflammatory activity leading to resolution of inflammation in several diseases.
Keywords: inflammation; macrophages; phenotype; photodynamic therapy and photothermal therapy.; theranostics.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





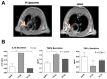
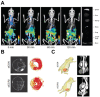

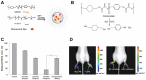
References
-
- Pene F, Courtine E, Cariou A, Mira JP. Toward theragnostics. Critical care medicine. 2009;37:S50–8. - PubMed
-
- Mura S, Couvreur P. Nanotheranostics for personalized medicine. Advanced drug delivery reviews. 2012;64:1394–416. - PubMed
-
- Couvreur P. Nanoparticles in drug delivery: past, present and future. Advanced drug delivery reviews. 2013;65:21–3. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

