Current strategies and challenges in engineering a bioartificial kidney
- PMID: 25553375
- PMCID: PMC4383659
- DOI: 10.2741/E729
Current strategies and challenges in engineering a bioartificial kidney
Abstract
Renal replacement therapy was an early pioneer in both extra-corporeal organ replacement and whole organ transplantation. Today, the success of this pioneering work is directly demonstrated in the millions of patients worldwide successfully treated with dialysis and kidney transplantation. However, there remain significant shortcomings to current treatment modalities that limit clinical outcomes and quality of life. To address these problems, researchers have turned to using cell-based therapies for the development of a bioartificial kidney. These approaches aim to recapitulate the numerous functions of the healthy kidney including solute clearance, fluid homeostasis and metabolic and endocrine functions. This review will examine the state-of-the-art in kidney bioengineering by evaluating the various techniques currently being utilized to create a bioartificial kidney. These promising new technologies, however, still need to address key issues that may limit the widespread adoption of cell therapy including cell sourcing, organ scaffolding, and immune response. Additionally, while these new methods have shown success in animal models, it remains to be seen whether these techniques can be successfully adapted for clinical treatment in humans.
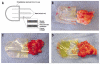
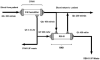
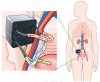
Figures





Similar articles
-
Bioengineering for Organ Transplantation: Progress and Challenges.Bioengineered. 2015;6(5):257-61. doi: 10.1080/21655979.2015.1081320. Epub 2015 Aug 11. Bioengineered. 2015. PMID: 26259720 Free PMC article. Review.
-
Regeneration and bioengineering of the kidney: current status and future challenges.Curr Urol Rep. 2014 Jan;15(1):379. doi: 10.1007/s11934-013-0379-9. Curr Urol Rep. 2014. PMID: 24375058 Review.
-
Bioengineering Organs for Blood Detoxification.Adv Healthc Mater. 2018 Nov;7(21):e1800430. doi: 10.1002/adhm.201800430. Epub 2018 Sep 19. Adv Healthc Mater. 2018. PMID: 30230709 Review.
-
The bioartificial kidney: current status and future promise.Pediatr Nephrol. 2014 Mar;29(3):343-51. doi: 10.1007/s00467-013-2467-y. Epub 2013 Apr 26. Pediatr Nephrol. 2014. PMID: 23619508 Review.
-
Bioengineering strategies for nephrologists: kidney was not built in a day.Expert Opin Biol Ther. 2020 May;20(5):467-480. doi: 10.1080/14712598.2020.1709439. Epub 2020 Jan 23. Expert Opin Biol Ther. 2020. PMID: 31971029 Review.
Cited by
-
Bioengineering for Organ Transplantation: Progress and Challenges.Bioengineered. 2015;6(5):257-61. doi: 10.1080/21655979.2015.1081320. Epub 2015 Aug 11. Bioengineered. 2015. PMID: 26259720 Free PMC article. Review.
-
Bioartificial Kidneys.Curr Stem Cell Rep. 2017 Jun;3(2):68-76. doi: 10.1007/s40778-017-0079-3. Epub 2017 Apr 12. Curr Stem Cell Rep. 2017. PMID: 33005562 Free PMC article.
-
Novel surgical techniques, regenerative medicine, tissue engineering and innovative immunosuppression in kidney transplantation.Arch Med Sci. 2016 Oct 1;12(5):1158-1173. doi: 10.5114/aoms.2016.61919. Epub 2016 Aug 25. Arch Med Sci. 2016. PMID: 27695507 Free PMC article.
-
Contemporary Perspectives on Chronic Renal Disorders.Chronic Dis Transl Med. 2025 Apr 17;11(2):89-104. doi: 10.1002/cdt3.70004. eCollection 2025 Jun. Chronic Dis Transl Med. 2025. PMID: 40486956 Free PMC article. Review.
-
Fabrication of Kidney Proximal Tubule Grafts Using Biofunctionalized Electrospun Polymer Scaffolds.Macromol Biosci. 2019 Feb;19(2):e1800412. doi: 10.1002/mabi.201800412. Epub 2018 Dec 13. Macromol Biosci. 2019. PMID: 30548802 Free PMC article.
References
-
- ESRD Patients in 2011 A Global Perspective. 2011
-
- USRDS Annual Data Report 2013. 2013
-
- Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, Kimata N, Gillespie BW, Combe C, Bommer J, Akiba T, Mapes DL, Young EW, Port FK. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int. 2006;69:1222–1228. 10.1038/sj.ki.5000186. - PubMed
-
- Buoncristiani U. Fifteen years of clinical experience with daily haemodialysis. Nephrol Dial Transplant. 1998;13(Suppl 6):148–151. - PubMed
-
- Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS. FHN Trial Grp: In-Center Hemodialysis Six Times per Week versus Three Times per Week. N Engl J Med. 2010;363:2287–2300. 10.1056/NEJMoa1001593. - PMC - PubMed

