Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial
- PMID: 25554404
- PMCID: PMC4361281
- DOI: 10.1016/S1474-4422(14)70318-7
Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial
Erratum in
-
Corrections.Lancet Neurol. 2015 Feb;14(2):135. doi: 10.1016/S1474-4422(15)70002-5. Lancet Neurol. 2015. PMID: 25772886 No abstract available.
Abstract
Background: Cardiomyopathy is a leading cause of death in patients with Duchenne muscular dystrophy and myocardial damage precedes decline in left ventricular systolic function. We tested the efficacy of eplerenone on top of background therapy in patients with Duchenne muscular dystrophy with early myocardial disease.
Methods: In this randomised, double-blind, placebo-controlled trial, boys from three centres in the USA aged 7 years or older with Duchenne muscular dystrophy, myocardial damage by late gadolinium enhancement cardiac MRI and preserved ejection fraction received either eplerenone 25 mg or placebo orally, every other day for the first month and once daily thereafter, in addition to background clinician-directed therapy with either angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB). Computer-generated randomisation was done centrally using block sizes of four and six, and only the study statistician and the investigational pharmacy had the preset randomisation assignments. The primary outcome was change in left ventricular circumferential strain (Ecc) at 12 months, a measure of contractile dysfunction. Safety was established through serial serum potassium levels and measurement of cystatin C, a non-creatinine measure of kidney function. This trial is registered with ClinicalTrials.gov, number NCT01521546.
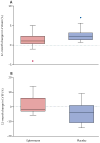
Findings: Between Jan 26, 2012, and July 3, 2013, 188 boys were screened and 42 were enrolled. 20 were randomly assigned to receive eplerenone and 22 to receive placebo, of whom 20 in the eplerenone group and 20 in the placebo group completed baseline, 6-month, and 12-month visits. After 12 months, decline in left ventricular circumferential strain was less in those who received eplerenone than in those who received placebo (median ΔEcc 1·0 [IQR 0·3-2·2] vs 2·2 [1·3-3·1]; p=0·020). Cystatin C concentrations remained normal in both groups, and all non-haemolysed blood samples showed normal potassium concentrations. One 23-year-old patient in the placebo group died of fat embolism, and another patient in the placebo group withdrew from the trial to address long-standing digestive issues. All other adverse events were mild: short-lived headaches coincident with seasonal allergies occurred in one patient given eplerenone, flushing occurred in one patient given placebo, and anxiety occurred in another patient given placebo.
Interpretation: In boys with Duchenne muscular dystrophy and preserved ejection fraction, addition of eplerenone to background ACEI or ARB therapy attenuates the progressive decline in left ventricular systolic function. Early use of available drugs warrants consideration in this population at high risk of cardiac death, but further studies are needed to determine the effect of combination cardioprotective therapy on event-free survival in Duchenne muscular dystrophy.
Funding: BallouSkies, Parent Project for Muscular Dystrophy, US National Center for Advancing Translational Sciences, and US National Institutes of Health.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Prevention of cardiomyopathy in Duchenne muscular dystrophy.Lancet Neurol. 2015 Feb;14(2):127-8. doi: 10.1016/S1474-4422(14)70326-6. Epub 2014 Dec 30. Lancet Neurol. 2015. PMID: 25554403 No abstract available.
References
-
- Romfh A, McNally EM. Cardiac assessment in Duchenne and Becker muscular dystrophies. Curr Heart Fail Rep. 2010;7:212–28. - PubMed
-
- Grothues F, Smith G, Moon J, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. - PubMed
-
- Hor KN, Mazur W, Taylor MD, et al. Effects of steroids and angiotensin converting enzyme inhibition on circumferential strain in boys with Duchenne muscular dystrophy: a cross-sectional and longitudinal study utilizing cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:60. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

