Biological Activity of N-Hydroxyethyl-4-aza-2,3-didehydropodophyllotoxin Derivatives upon Colorectal Adenocarcinoma Cells
- PMID: 25554737
- PMCID: PMC4279218
- DOI: 10.4236/ojmc.2014.41001
Biological Activity of N-Hydroxyethyl-4-aza-2,3-didehydropodophyllotoxin Derivatives upon Colorectal Adenocarcinoma Cells
Abstract
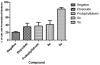
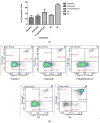
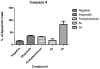
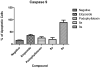
Etoposide is a chemotherapy drug derived from the natural lignin podophyllotoxin. Our novel generated Aza-podophyllotoxin compounds (AZP 8a & AZP 9a) are analogues of podophyllotoxin and were previously screened for anti-cancer activity through the NCI 60 cell line screening panel showing activity on various cell types including colon cancer. This study expands the toxicological screening by studying apoptosis and various hallmark events as part of the mechanism of action of these compounds on colon cancer cells. The COLO 205 cell line was selected and exposed to AZP to determine the IC50 doses at 24 hours treatment. Apoptosis hallmark events such as migration of phosphatidylserine (PS) to the cell membrane, DNA fragmentation, cell cycle effects, mitochondrial membrane permeabilization and caspase activation were included. Experiments were performed in triplicates for all tested compounds including AZP 8a, AZP 9a, camptothecin as positive control and vehicle as negative control. Our results present contrasting apoptotic activity between the experimental compounds. Compound 8a presented migration of PS (annexin V assay), DNA fragmentation and cell cycle arrest at S phase. Compound 9a presented PS migration with fragmented DNA, cell cycle arrest at S phase, mitochondrial membrane permeabilization and activation of caspase 3, 8 and 9. Compound 8a without the oxygen atoms in ring A appears to cause effects similarly to autophagy as induced by etoposide, a cancer drug analogue of our heterocyclic compounds. Compound 9a with the oxygen atoms in expanded ring A presented induction of cell death following activation of a classical apoptosis pathway. Our results suggest that minor structural differences among these AZP can account for the difference in biological response and cancer cell toxicity.
Keywords: Aza-Podophyllotoxin; COLO 205; Colon Cancer; Etoposide; Podophyllotoxin.
Figures


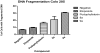

References
-
- Nagar N, Jat R, Saharan R, Verma S, Sharma D, Bansal K. Podophyllotoxin and Their Glicosidic Derivatives. Pharmacophore. 2011;2:124–134.
-
- Kaplan IW. Codylomata acuminata. The New Orleans Medical and Surgical Journal. 1942;94:388.
-
- Liu YQ, Yang LM, Tian X. Podophyllotoxin: Current Perspectives. Current Bioactive Compounds. 2007;3:37–66. http://dx.doi.org/10.2174/157340707780126499. - DOI
-
- Wang RW, Rebhum LI, Kupchan SM. Anti Mitotic and Antitubulin Activity of the Tumor Inhibitor Steganacin. Cancer Research. 1977;37:3071–3079. - PubMed
-
- Stähelin HF, Von Wartburg A, Stã HF. The Chemical and Biological Route from Podophyllotoxin Glucoside to Etoposide: Ninth Cain Memorial Award Lecture The Chemical and Biological Route from Podophyllotoxin Glucoside to Etoposide. Ninth Cain Memorial Award Lecture. 1991;1:5–15. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
