Identification of a conserved set of upregulated genes in mouse skeletal muscle hypertrophy and regrowth
- PMID: 25554798
- PMCID: PMC4347749
- DOI: 10.1152/japplphysiol.00351.2014
Identification of a conserved set of upregulated genes in mouse skeletal muscle hypertrophy and regrowth
Abstract
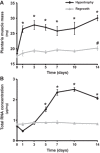
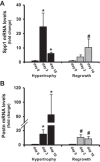
The purpose of this study was to compare the gene expression profile of mouse skeletal muscle undergoing two forms of growth (hypertrophy and regrowth) with the goal of identifying a conserved set of differentially expressed genes. Expression profiling by microarray was performed on the plantaris muscle subjected to 1, 3, 5, 7, 10, and 14 days of hypertrophy or regrowth following 2 wk of hind-limb suspension. We identified 97 differentially expressed genes (≥2-fold increase or ≥50% decrease compared with control muscle) that were conserved during the two forms of muscle growth. The vast majority (∼90%) of the differentially expressed genes was upregulated and occurred at a single time point (64 out of 86 genes), which most often was on the first day of the time course. Microarray analysis from the conserved upregulated genes showed a set of genes related to contractile apparatus and stress response at day 1, including three genes involved in mechanotransduction and four genes encoding heat shock proteins. Our analysis further identified three cell cycle-related genes at day and several genes associated with extracellular matrix (ECM) at both days 3 and 10. In conclusion, we have identified a core set of genes commonly upregulated in two forms of muscle growth that could play a role in the maintenance of sarcomere stability, ECM remodeling, cell proliferation, fast-to-slow fiber type transition, and the regulation of skeletal muscle growth. These findings suggest conserved regulatory mechanisms involved in the adaptation of skeletal muscle to increased mechanical loading.
Keywords: extracellular matrix; hind-limb suspension; mechanotransduction; stress response; transcriptome.
Copyright © 2015 the American Physiological Society.
Figures



Similar articles
-
Changes in muscle fiber contractility and extracellular matrix production during skeletal muscle hypertrophy.J Appl Physiol (1985). 2017 Mar 1;122(3):571-579. doi: 10.1152/japplphysiol.00719.2016. Epub 2016 Dec 15. J Appl Physiol (1985). 2017. PMID: 27979985 Free PMC article.
-
Time course of gene expression during mouse skeletal muscle hypertrophy.J Appl Physiol (1985). 2013 Oct 1;115(7):1065-74. doi: 10.1152/japplphysiol.00611.2013. Epub 2013 Jul 18. J Appl Physiol (1985). 2013. PMID: 23869057 Free PMC article.
-
Expression Levels of Long Non-Coding RNAs Change in Models of Altered Muscle Activity and Muscle Mass.Int J Mol Sci. 2020 Feb 27;21(5):1628. doi: 10.3390/ijms21051628. Int J Mol Sci. 2020. PMID: 32120896 Free PMC article.
-
MicroRNAs in skeletal and cardiac muscle development.DNA Cell Biol. 2007 Apr;26(4):219-25. doi: 10.1089/dna.2006.0556. DNA Cell Biol. 2007. PMID: 17465888 Review.
-
Disrupted autophagy undermines skeletal muscle adaptation and integrity.Mamm Genome. 2016 Dec;27(11-12):525-537. doi: 10.1007/s00335-016-9659-2. Epub 2016 Aug 2. Mamm Genome. 2016. PMID: 27484057 Free PMC article. Review.
Cited by
-
Changes in muscle fiber contractility and extracellular matrix production during skeletal muscle hypertrophy.J Appl Physiol (1985). 2017 Mar 1;122(3):571-579. doi: 10.1152/japplphysiol.00719.2016. Epub 2016 Dec 15. J Appl Physiol (1985). 2017. PMID: 27979985 Free PMC article.
-
Dysregulation of lipid metabolism and appearance of slow myofiber-specific isoforms accompany the development of Wooden Breast myopathy in modern broiler chickens.Sci Rep. 2019 Nov 20;9(1):17170. doi: 10.1038/s41598-019-53728-8. Sci Rep. 2019. PMID: 31748687 Free PMC article.
-
Exercise Capacity and Response to Training Quantitative Trait Loci in a NZW X 129S1 Intercross and Combined Cross Analysis of Inbred Mouse Strains.PLoS One. 2015 Dec 28;10(12):e0145741. doi: 10.1371/journal.pone.0145741. eCollection 2015. PLoS One. 2015. PMID: 26710100 Free PMC article.
-
Platelet-Rich Plasma Activates Proinflammatory Signaling Pathways and Induces Oxidative Stress in Tendon Fibroblasts.Am J Sports Med. 2016 Aug;44(8):1931-40. doi: 10.1177/0363546516637176. Epub 2016 Jul 8. Am J Sports Med. 2016. PMID: 27400714 Free PMC article.
-
Hippo Pathway and Skeletal Muscle Mass Regulation in Mammals: A Controversial Relationship.Front Physiol. 2017 Mar 29;8:190. doi: 10.3389/fphys.2017.00190. eCollection 2017. Front Physiol. 2017. PMID: 28424630 Free PMC article. Review.
References
-
- Adams GR, Haddad F, Baldwin KM. Time course of changes in markers of myogenesis in overloaded rat skeletal muscles. J Appl Physiol 87: 1705–1712, 1999. - PubMed
-
- Barash IA, Bang ML, Mathew L, Greaser ML, Chen J, Lieber RL. Structural and regulatory roles of muscle ankyrin repeat protein family in skeletal muscle. Am J Physiol Cell Physiol 293: C218–C227, 2007. - PubMed
-
- Barash IA, Mathew L, Lahey M, Greaser ML, Lieber RL. Muscle LIM protein plays both structural and functional roles in skeletal muscle. Am J Physiol Cell Physiol 289: C1312–C1320, 2005. - PubMed
-
- Barash IA, Mathew L, Ryan AF, Chen J, Lieber RL. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol 286: C355–C364, 2004. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

