Serum protein layers on parylene-C and silicon oxide: effect on cell adhesion
- PMID: 25555155
- PMCID: PMC4342411
- DOI: 10.1016/j.colsurfb.2014.12.020
Serum protein layers on parylene-C and silicon oxide: effect on cell adhesion
Abstract
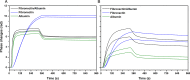
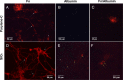
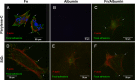
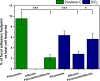
Among the range of materials used in bioengineering, parylene-C has been used in combination with silicon oxide and in presence of the serum proteins, in cell patterning. However, the structural properties of adsorbed serum proteins on these substrates still remain elusive. In this study, we use an optical biosensing technique to decipher the properties of fibronectin (Fn) and serum albumin adsorbed on parylene-C and silicon oxide substrates. Our results show the formation of layers with distinct structural and adhesive properties. Thin, dense layers are formed on parylene-C, whereas thicker, more diffuse layers are formed on silicon oxide. These results suggest that Fn acquires a compact structure on parylene-C and a more extended structure on silicon oxide. Nonetheless, parylene-C and silicon oxide substrates coated with Fn host cell populations that exhibit focal adhesion complexes and good cell attachment. Albumin adopts a deformed structure on parylene-C and a globular structure on silicon oxide, and does not support significant cell attachment on either surface. Interestingly, the co-incubation of Fn and albumin at the ratio found in serum, results in the preferential adsorption of albumin on parylene-C and Fn on silicon oxide. This finding is supported by the exclusive formation of focal adhesion complexes in differentiated mouse embryonic stem cells (CGR8), cultured on Fn/albumin coated silicon oxide, but not on parylene-C. The detailed information provided in this study on the distinct properties of layers of serum proteins on substrates such as parylene-C and silicon oxide is highly significant in developing methods for cell patterning.
Keywords: Biosensing technique; Cell adhesion; Fibronectin; Parylene-C; Serum protein adsorption; Silicon oxide.
Copyright © 2014 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




References
-
- Herrault F., Yorish S., Crittenden T.M., Chang-Hyeon J., Allen M.G. Parylene-insulated ultradense microfabricated coils. J. Microelectromech. Syst. 2010;19:1277–1283.
-
- Temiz Y., Ferretti A., Leblebici Y., Guiducci C. A comparative study on fabrication techniques for on-chip microelectrodes. Lab Chip. 2012;12:4920–4928. - PubMed
-
- Goda T., Konno T., Takai M., Ishihara K. Photoinduced phospholipid polymer grafting on parylene film: advanced lubrication and antibiofouling properties. Colloids Surf. B: Biointerfaces. 2007;54:67–73. - PubMed
-
- Jui-Mei H., Rieth L., Normann R.A., Tathireddy P., Solzbacher F. Encapsulation of an integrated neural interface device with parylene C. IEEE Trans. Biomed. Eng. 2009;56:23–29. - PubMed
-
- Sharma A., Rieth L., Tathireddy P., Harrison R., Oppermann H., Klein M., Topper M., Jung E., Normann R., Clark G., Solzbacher F. Long term in vitro functional stability and recording longevity of fully integrated wireless neural interfaces based on the Utah Slant Electrode Array. J. Neural Eng. 2011;8:045004. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

