TLR4 genotype and environmental LPS mediate RSV bronchiolitis through Th2 polarization
- PMID: 25555213
- PMCID: PMC4319428
- DOI: 10.1172/JCI75183
TLR4 genotype and environmental LPS mediate RSV bronchiolitis through Th2 polarization
Abstract
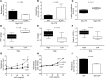
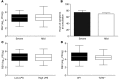
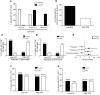
While 30%-70% of RSV-infected infants develop bronchiolitis, 2% require hospitalization. It is not clear why disease severity differs among healthy, full-term infants; however, virus titers, inflammation, and Th2 bias are proposed explanations. While TLR4 is associated with these disease phenotypes, the role of this receptor in respiratory syncytial virus (RSV) pathogenesis is controversial. Here, we evaluated the interaction between TLR4 and environmental factors in RSV disease and defined the immune mediators associated with severe illness. Two independent populations of infants with RSV bronchiolitis revealed that the severity of RSV infection is determined by the TLR4 genotype of the individual and by environmental exposure to LPS. RSV-infected infants with severe disease exhibited a high GATA3/T-bet ratio, which manifested as a high IL-4/IFN-γ ratio in respiratory secretions. The IL-4/IFN-γ ratio present in infants with severe RSV is indicative of Th2 polarization. Murine models of RSV infection confirmed that LPS exposure, Tlr4 genotype, and Th2 polarization influence disease phenotypes. Together, the results of this study identify environmental and genetic factors that influence RSV pathogenesis and reveal that a high IL-4/IFN-γ ratio is associated with severe disease. Moreover, these molecules should be explored as potential targets for therapeutic intervention.
Figures


References
-
- Collins PL, Chanock RM, Murphy BR. Respiratory syncytial virus disease. In: Knipe D, et al., eds. Fields Virology. 4th ed. Vol.1. Philadelphia, Pennsylvania, USA: Lippincott Williams & Wilkins; 2001:1443–1486.
-
- Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143(5 suppl):S118–S126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

