Inositol 1,4,5-trisphosphate receptors in the endoplasmic reticulum: A single-channel point of view
- PMID: 25555684
- PMCID: PMC4458407
- DOI: 10.1016/j.ceca.2014.12.008
Inositol 1,4,5-trisphosphate receptors in the endoplasmic reticulum: A single-channel point of view
Abstract
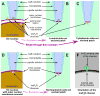
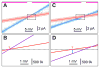
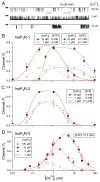
As an intracellular Ca(2+) release channel at the endoplasmic reticulum membrane, the ubiquitous inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R) plays a crucial role in the generation, propagation and regulation of intracellular Ca(2+) signals that regulate numerous physiological and pathophysiological processes. This review provides a concise account of the fundamental single-channel properties of the InsP3R channel: its conductance properties and its regulation by InsP3 and Ca(2+), its physiological ligands, studied using nuclear patch clamp electrophysiology.
Keywords: Calcium signal; Endoplasmic reticulum; Inositol 1,4,5-trisphosphate receptor; Intracellular calcium; Ion channel; Patch clamp electrophysiology.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures



References
-
- Putney JW, Jr, Bird GS. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr Rev. 1993;14:610–631. - PubMed
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

