Subunit-specific regulation of N-methyl-D-aspartate (NMDA) receptor trafficking by SAP102 protein splice variants
- PMID: 25555912
- PMCID: PMC4335245
- DOI: 10.1074/jbc.M114.599969
Subunit-specific regulation of N-methyl-D-aspartate (NMDA) receptor trafficking by SAP102 protein splice variants
Abstract
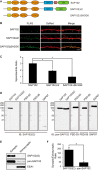
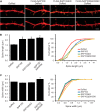
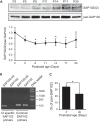
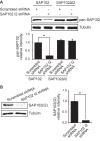
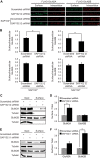
Synapse-associated protein 102 (SAP102) is a scaffolding protein abundantly expressed early in development that mediates glutamate receptor trafficking during synaptogenesis. Mutations in human SAP102 have been reported to cause intellectual disability, which is consistent with its important role during early postnatal development. SAP102 contains PDZ, SH3, and guanylate kinase (GK)-like domains, which mediate specific protein-protein interactions. SAP102 binds directly to N-methyl-D-aspartate receptors (NMDARs), anchors receptors at synapses, and facilitates transduction of NMDAR signals. Proper localization of SAP102 at the postsynaptic density is essential to these functions. However, how SAP102 is targeted to synapses is unclear. In the current study we find that synaptic localization of SAP102 is regulated by alternative splicing. The SAP102 splice variant that possesses a C-terminal insert (I2) between the SH3 and GK domains is highly enriched at dendritic spines. We also show that there is an intramolecular interaction between the SH3 and GK domains in SAP102 but that the I2 splicing does not influence SH3-GK interaction. Previously, we have shown that SAP102 expression promotes spine lengthening. We now find that the spine lengthening effect is independent of the C-terminal alternative splicing of SAP102. In addition, expression of I2-containing SAP102 isoforms is regulated developmentally. Knockdown of endogenous I2-containing SAP102 isoforms differentially affect NMDAR surface expression in a subunit-specific manner. These data shed new light on the role of SAP102 in the regulation of NMDAR trafficking.
Keywords: Glutamate Receptor; MAGUK; NMDA; PDZ Domain; Protein Splicing; Protein Targeting; SAP102; Scaffold Protein.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Elias G. M., Nicoll R. A. (2007) Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 17, 343–352 - PubMed
-
- El-Husseini A. E., Topinka J. R., Lehrer-Graiwer J. E., Firestein B. L., Craven S. E., Aoki C., Bredt D. S. (2000) Ion channel clustering by membrane-associated guanylate kinases. Differential regulation by N-terminal lipid and metal binding motifs. J. Biol. Chem. 275, 23904–23910 - PubMed
-
- Cuthbert P. C., Stanford L. E., Coba M. P., Ainge J. A., Fink A. E., Opazo P., Delgado J. Y., Komiyama N. H., O'Dell T. J., Grant S. G. (2007) Synapse-associated protein 102/dlgh3 couples the NMDA receptor to specific plasticity pathways and learning strategies. J. Neurosci. 27, 2673–2682 - PMC - PubMed
-
- Sans N., Prybylowski K., Petralia R. S., Chang K., Wang Y. X., Racca C., Vicini S., Wenthold R. J. (2003) NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat. Cell Biol. 5, 520–530 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

