Inability to maintain GSH pool in G6PD-deficient red cells causes futile AMPK activation and irreversible metabolic disturbance
- PMID: 25556665
- PMCID: PMC4361223
- DOI: 10.1089/ars.2014.6142
Inability to maintain GSH pool in G6PD-deficient red cells causes futile AMPK activation and irreversible metabolic disturbance
Abstract
Aims: Glucose 6-phosphate dehydrogenase (G6PD) is essential for maintenance of nicotinamide dinucleotide hydrogen phosphate (NADPH) levels and redox homeostasis. A number of drugs, such as antimalarial drugs, act to induce reactive oxygen species and hemolytic crisis in G6PD-deficient patients. We used diamide (DIA) to mimic drug-induced oxidative stress and studied how these drugs affect cellular metabolism using a metabolomic approach.
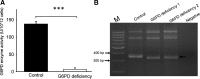
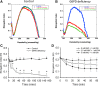
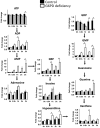
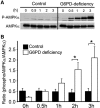
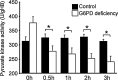
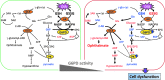
Results: There are a few differences in metabolome between red blood cells (RBCs) from normal and G6PD-deficient individuals. DIA causes modest changes in normal RBC metabolism. In contrast, there are significant changes in various biochemical pathways, namely glutathione (GSH) metabolism, purine metabolism, and glycolysis, in G6PD-deficient cells. GSH depletion is concomitant with a shift in energy metabolism. Adenosine monophosphate (AMP) and adenosine diphosphate (ADP) accumulation activates AMP protein kinase (AMPK) and increases entry of glucose into glycolysis. However, inhibition of pyruvate kinase (PK) reduces the efficacy of energy production. Metabolic changes and protein oxidation occurs to a greater extent in G6PD-deficient RBCs than in normal cells, leading to severe irreversible loss of deformability of the former.
Innovation and conclusion: Normal and G6PD-deficient RBCs differ in their responses to oxidants. Normal cells have adequate NADPH regeneration for maintenance of GSH pool. In contrast, G6PD-deficient cells are unable to regenerate enough NADPH under a stressful situation, and switch to biosynthetic pathway for GSH supply. Rapid GSH exhaustion causes energy crisis and futile AMPK activation. Our findings suggest that drug-induced oxidative stress differentially affects metabolism and metabolite signaling in normal and G6PD-deficient cells. It also provides an insight into the pathophysiology of acute hemolytic anemia in G6PD-deficient patients.
Figures



References
-
- Agarwal KC, Miech RP, and Parks RE., Jr.Guanylate kinases from human erythrocytes, hog brain, and rat liver. Methods Enzymol 51: 483–490, 1978 - PubMed
-
- Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, and Cantley LC. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334: 1278–1283, 2011 - PMC - PubMed
-
- Auth T, Safran SA, and Gov NS. Fluctuations of coupled fluid and solid membranes with application to red blood cells. Phys Rev E Stat Nonlin Soft Matter Phys 76: 051910, 2007 - PubMed
-
- Ballatori N. and Dutczak WJ. Identification and characterization of high and low affinity transport systems for reduced glutathione in liver cell canalicular membranes. J Biol Chem 269: 19731–19737, 1994 - PubMed
-
- Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, and Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK). J Cell Sci 115: 2433–2442, 2002 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

