A Convenient and Efficient Method to Enrich and Maintain Highly Proliferative Human Fetal Liver Stem Cells
- PMID: 25556695
- PMCID: PMC4491163
- DOI: 10.1089/rej.2014.1619
A Convenient and Efficient Method to Enrich and Maintain Highly Proliferative Human Fetal Liver Stem Cells
Abstract
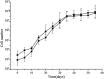
Pluripotent human hepatic stem cells have broad research and clinical applications, which are, however, restricted by both limited resources and technical difficulties with respect to isolation of stem cells from the adult or fetal liver. In this study, we developed a convenient and efficient method involving a two-step in situ collagenase perfusion, gravity sedimentation, and Percoll density gradient centrifugation to enrich and maintain highly proliferative human fetal liver stem cells (hFLSCs). Using this method, the isolated hFLSCs entered into the exponential growth phase within 10 days and maintained sufficient proliferative activity to permit subculture for at least 20 passages without differentiation. Immunocytochemistry, immunofluorescence, and flow cytometry results showed that these cells expressed stem cell markers, such as c-kit, CD44, epithelial cell adhesion molecule (EpCAM), oval cell marker-6 (OV-6), epithelial marker cytokeratin 18 (CK18), biliary ductal marker CK19, and alpha-fetoprotein (AFP). Gene expression analysis showed that these cells had stable mRNA expression of c-Kit, EpCAM, neural cell adhesion molecule (NCAM), CK19, CK18, AFP, and claudin 3 (CLDN-3) throughout each passage while maintaining low levels of ALB, but with complete absence of cytochrome P450 3A4 (C3A4), phosphoenolpyruvate carboxykinase (PEPCK), telomeric repeat binding factor (TRF), and connexin 26 (CX26) expression. When grown in appropriate medium, these isolated liver stem cells could differentiate into hepatocytes, cholangiocytes, osteoblasts, adipocytes, or endothelial cells. Thus, we have demonstrated a more economical and efficient method to isolate hFLSCs than magnetic-activated cell sorting (MACS). This novel approach may provide an excellent tool to isolate highly proliferative hFLSCs for tissue engineering and regenerative therapies.
Figures


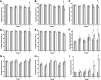
 ) Significant statistical difference (p<0.05) of the expression of cell-surface markers for these two methods.
) Significant statistical difference (p<0.05) of the expression of cell-surface markers for these two methods.


References
-
- Khan AA, Shaik MV, Parveen N, Rajendraprasad A, Aleem MA, Habeeb MA, Srinivas G, Raj TA, Tiwari SK, Kumaresan K, Venkateswarlu J, Pande G, Habibullah CM. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant 2010;19:409–418 - PubMed
-
- Defresne F, Tondreau T, Stephenne X, Smets F, Bourgois A, Najimi M, Jamar F, Sokal EM. Biodistribution of adult derived human liver stem cells following intraportal infusion in a 17-year-old patient with glycogenosis type 1A. Nucl Med Biol 2014;41:371–375 - PubMed
-
- Pietrosi G, Vizzini G, Gerlach J, Chinnici C, Luca A, Amico G, M DA, Conaldi PG, Petri SL, Spada M, Tuzzolino F, Alio L, Schmelzer E, Gridelli B. Phase I–II matched case-control study of human fetal liver cell transplantation for treatment of chronic liver disease. Cell Transplant 2014; doi: 10.3727/096368914X682422 - DOI - PubMed
-
- Oertel M. Fetal liver cell transplantation as a potential alternative to whole liver transplantation? J Gastroenterol 2011;46:953–965 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

