Isolevuglandin adducts in disease
- PMID: 25557218
- PMCID: PMC4484713
- DOI: 10.1089/ars.2014.6154
Isolevuglandin adducts in disease
Abstract
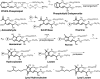
Significance: A diverse family of lipid-derived levulinaldehydes, isolevuglandins (isoLGs), is produced by rearrangement of endoperoxide intermediates generated through both cyclooxygenase (COX) and free radical-induced cyclooxygenation of polyunsaturated fatty acids and their phospholipid esters. The formation and reactions of isoLGs with other biomolecules has been linked to alcoholic liver disease, Alzheimer's disease, age-related macular degeneration, atherosclerosis, cardiac arythmias, cancer, end-stage renal disease, glaucoma, inflammation of allergies and infection, mitochondrial dysfunction, multiple sclerosis, and thrombosis. This review chronicles progress in understanding the chemistry of isoLGs, detecting their production in vivo and understanding their biological consequences.
Critical issues: IsoLGs have never been isolated from biological sources, because they form adducts with primary amino groups of other biomolecules within seconds. Chemical synthesis enabled investigation of isoLG chemistry and detection of isoLG adducts present in vivo.
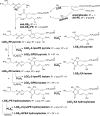
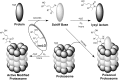
Recent advances: The first peptide mapping and sequencing of an isoLG-modified protein present in human retina identified the modification of a specific lysyl residue of the sterol C27-hydroxylase Cyp27A1. This residue is preferentially modified by iso[4]LGE2 in vitro, causing loss of function. Adduction of less than one equivalent of isoLG can induce COX-associated oligomerization of the amyloid peptide Aβ1-42. Adduction of isoLGE2 to phosphatidylethanolamines causes gain of function, converting them into proinflammatory isoLGE2-PE agonists that foster monocyte adhesion to endothelial cells.
Future directions: Among the remaining questions on the biochemistry of isoLGs are the dependence of biological activity on isoLG isomer structure, the structures and mechanism of isoLG-derived protein-protein and DNA-protein cross-link formation, and its biological consequences.
Figures





References
-
- Amarnath V, Amarnath K, Amarnath K, Davies S, and Roberts LJ., 2nd Pyridoxamine: an extremely potent scavenger of 1,4-dicarbonyls. Chem Res Toxicol 17: 410–415, 2004 - PubMed
-
- Bernoud-Hubac N, Alam DA, Lefils J, Davies SS, Amarnath V, Guichardant M, Roberts LJ, 2nd, and Lagarde M. Low concentrations of reactive gamma-ketoaldehydes prime thromboxane-dependent human platelet aggregation via p38-MAPK activation. Biochim Biophys Acta 1791: 307–313, 2009 - PubMed
-
- Bernoud-Hubac N, Davies SS, Boutaud O, Montine TJ, and Roberts LJ., 2nd Formation of highly reactive gamma-ketoaldehydes (neuroketals) as products of the neuroprostane pathway. J Biol Chem 276: 30964–30970, 2001 - PubMed
-
- Bernoud-Hubac N, Fay LB, Armarnath V, Guichardant M, Bacot S, Davies SS, Roberts LJ, 2nd, and Lagarde M. Covalent binding of isoketals to ethanolamine phospholipids. Free Radic Biol Med 37: 1604–1611, 2004 - PubMed
-
- Bernoud-Hubac N. and Roberts LJ., 2nd Identification of oxidized derivatives of neuroketals. Biochemistry 41: 11466–11471, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

