AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis
- PMID: 25557369
- PMCID: PMC4308718
- DOI: 10.1038/ncomms6928
AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis
Abstract
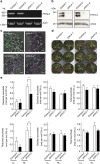
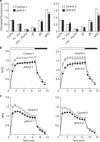
Ascorbate is an antioxidant and coenzyme for various metabolic reactions in vivo. In plant chloroplasts, high ascorbate levels are required to overcome photoinhibition caused by strong light. However, ascorbate is synthesized in the mitochondria and the molecular mechanisms underlying ascorbate transport into chloroplasts are unknown. Here we show that AtPHT4;4, a member of the phosphate transporter 4 family of Arabidopsis thaliana, functions as an ascorbate transporter. In vitro analysis shows that proteoliposomes containing the purified AtPHT4;4 protein exhibit membrane potential- and Cl(-)-dependent ascorbate uptake. The AtPHT4;4 protein is abundantly expressed in the chloroplast envelope membrane. Knockout of AtPHT4;4 results in decreased levels of the reduced form of ascorbate in the leaves and the heat dissipation process of excessive energy during photosynthesis is compromised. Taken together, these observations indicate that the AtPHT4;4 protein is an ascorbate transporter at the chloroplast envelope membrane, which may be required for tolerance to strong light stress.
Figures





Similar articles
-
Redox regulation of ascorbate and glutathione by a chloroplastic dehydroascorbate reductase is required for high-light stress tolerance in Arabidopsis.Biosci Biotechnol Biochem. 2016 May;80(5):870-7. doi: 10.1080/09168451.2015.1135042. Epub 2016 Mar 1. Biosci Biotechnol Biochem. 2016. PMID: 26927949
-
SULTR3;1 is a chloroplast-localized sulfate transporter in Arabidopsis thaliana.Plant J. 2013 Feb;73(4):607-16. doi: 10.1111/tpj.12059. Epub 2012 Dec 28. Plant J. 2013. PMID: 23095126
-
Decreased capacity for sodium export out of Arabidopsis chloroplasts impairs salt tolerance, photosynthesis and plant performance.Plant J. 2014 May;78(4):646-58. doi: 10.1111/tpj.12501. Plant J. 2014. PMID: 24617758
-
Ions channels/transporters and chloroplast regulation.Cell Calcium. 2015 Jul;58(1):86-97. doi: 10.1016/j.ceca.2014.10.002. Epub 2014 Oct 13. Cell Calcium. 2015. PMID: 25454594 Review.
-
Targeting and biogenesis of transporters and channels in chloroplast envelope membranes: Unsolved questions.Cell Calcium. 2015 Jul;58(1):122-30. doi: 10.1016/j.ceca.2014.10.012. Epub 2014 Oct 31. Cell Calcium. 2015. PMID: 25465895 Review.
Cited by
-
Chloroplast phosphate transporter CrPHT4-7 regulates phosphate homeostasis and photosynthesis in Chlamydomonas.Plant Physiol. 2024 Feb 29;194(3):1646-1661. doi: 10.1093/plphys/kiad607. Plant Physiol. 2024. PMID: 37962583 Free PMC article.
-
Phosphate Uptake and Allocation - A Closer Look at Arabidopsis thaliana L. and Oryza sativa L.Front Plant Sci. 2016 Aug 15;7:1198. doi: 10.3389/fpls.2016.01198. eCollection 2016. Front Plant Sci. 2016. PMID: 27574525 Free PMC article. Review.
-
Interdependent iron and phosphorus availability controls photosynthesis through retrograde signaling.Nat Commun. 2021 Dec 10;12(1):7211. doi: 10.1038/s41467-021-27548-2. Nat Commun. 2021. PMID: 34893639 Free PMC article.
-
Comprehensive Genomic Identification and Expression Analysis of the Phosphate Transporter (PHT) Gene Family in Apple.Front Plant Sci. 2017 Mar 30;8:426. doi: 10.3389/fpls.2017.00426. eCollection 2017. Front Plant Sci. 2017. PMID: 28424713 Free PMC article.
-
The ascorbate biosynthesis pathway in plants is known, but there is a way to go with understanding control and functions.J Exp Bot. 2024 May 3;75(9):2604-2630. doi: 10.1093/jxb/erad505. J Exp Bot. 2024. PMID: 38300237 Free PMC article. Review.
References
-
- Englard S. & Seifter S. The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 6, 365–406 (1986). - PubMed
-
- Smirnoff N. & Wheeler G. L. Ascorbic acid in plants: biosynthesis and function. Crit. Rev. Biochem. Mol. Biol. 35, 291–314 (2000). - PubMed
-
- Gallie D. R. The role of L-ascorbic acid recycling in responding to environmental stress and in promoting plant growth. J. Exp. Bot. 64, 433–443 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

