Effects of castration on expression of lipid metabolism genes in the liver of korean cattle
- PMID: 25557684
- PMCID: PMC4283181
- DOI: 10.5713/ajas.14.0582
Effects of castration on expression of lipid metabolism genes in the liver of korean cattle
Abstract
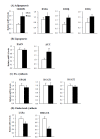
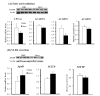
Castration induces the accumulation of body fat and deposition of intramuscular fat in Korean cattle, resulting in improved beef quality. However, little is known about the metabolic adaptations in the liver following castration. To understand changes in lipid metabolism following castration, hepatic expression levels of lipid metabolism genes were compared between Korean bulls and steers. Steers had higher (p<0.001) hepatic lipids contents and higher (p<0.01) mRNA levels of lipogenic acetyl-CoA carboxylase. This differential gene expression may, in part, contribute to increased hepatic lipid content following the castration of bulls. However, we found no differences in the hepatic expression levels of genes related to triglyceride synthesis (mitochondrial glycerol-3-phosphate acyltransferase, diacylglycerol O-acyltransferase 1 and 2) and fatty acid (FA) oxidation (carnitine palmitoyltransferase 1A, C-4 to C-12 straight chain acyl-CoA dehydrogenase, very long chain acyl-CoA dehydrogenase) between bulls and steers. No differences in gene expression for very-low-density lipoprotein (VLDL) secretion, including apolipoprotein B mRNA and microsomal triglyceride transfer protein (MTTP) protein, were observed in the liver although MTTP mRNA levels were higher in steers compared to bulls. In conclusion, FA synthesis may contribute to increased hepatic lipid deposition in steers following castration. However, hepatic lipid metabolism, including triglyceride synthesis, FA oxidation, and VLDL secretion, was not significantly altered by castration. Our results suggest that hepatic lipid metabolism does not significantly contribute to increased body fat deposition in steers following castration.
Keywords: Bulls; Gene Expression; Korean Cattle; Liver; Steers.
Figures

References
-
- Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008;60:311–357. - PubMed
-
- Bonen A, Chabowski A, Luiken JJ, Glatz JF. Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms and regulation of protein-mediated cellular fatty acid uptake: Molecular, biochemical, and physiological evidence. Physiology (Bethesda, Md) 2007;22:15–29. - PubMed
-
- Bong JJ, Jeong JY, Rajasekar P, Cho YM, Kwon EG, Kim HC, Paek BH, Baik M. Differential expression of genes associated with lipid metabolism in longissimus dorsi of Korean bulls and steers. Meat Sci. 2012;91:284–293. - PubMed
-
- Chen X, Wang X, Li Z, Kong L, Liu G, Fu J, Wang A. Molecular cloning, tissue expression and protein structure prediction of the porcine 3-hydroxy-3-methylglutaryl-Coenzyme A reductase (HMGR) gene. Gene. 2012;495:170–177. - PubMed
-
- Edwards PA, Kennedy MA, Mak PA. LXRs; oxysterol-activated nuclear receptors that regulate genes controlling lipid homeostasis. Vascul Pharmacol. 2002;38:249–256. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

