The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era
- PMID: 25557753
- PMCID: PMC4485977
- DOI: 10.1002/ijc.29408
The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era
Abstract
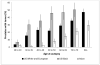
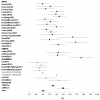
Widespread prostate-specific antigen (PSA) screening detects many cancers that would have otherwise gone undiagnosed. To estimate the prevalence of unsuspected prostate cancer, we reviewed 19 studies of prostate cancer discovered at autopsy among 6,024 men. Among men aged 70-79, tumor was found in 36% of Caucasians and 51% of African-Americans. This enormous prevalence, coupled with the high sensitivity of PSA screening, has led to the marked increase in the apparent incidence of prostate cancer. The impact of PSA screening on clinical practice is well-recognized, but its effect on epidemiologic research is less appreciated. Before screening, a larger proportion of incident prostate cancers had lethal potential and were diagnosed at advanced stage. However, in the PSA era, overall incident prostate cancer mainly is indolent disease, and often reflects the propensity to be screened and biopsied. Studies must therefore focus on cancers with lethal potential, and include long follow-up to accommodate the lead time induced by screening. Moreover, risk factor patterns differ markedly for potentially lethal and indolent disease, suggesting separate etiologies and distinct disease entities. Studies of total incident or indolent prostate cancer are of limited clinical utility, and the main focus of research should be on prostate cancers of lethal potential.
Keywords: PSA-screening; autopsy; epidemiology; lethal prostate cancer; prostate cancer.
© 2014 UICC.
Figures

References
-
- Mariotto AB, Etzioni R, Krapcho M, Feuer EJ. Reconstructing PSA testing patterns between black and white men in the US from Medicare claims and the National Health Interview Survey. Cancer. 2007;109:1877–86. - PubMed
-
- Draisma G, Boer R, Otto SJ, van der Cruijsen IW, Damhuis RAM, Schröder FH, de Koning HJ. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. Journal of the National Cancer Institute. 2003;95:868–78. - PubMed
-
- Auvinen A, Määttänen L, Stenman U-H, Tammela T, Rannikko S, Aro J, Juusela H, Hakama M. Lead-time in prostate cancer screening (Finland) Cancer Causes and Control. 2002;13:279–85. - PubMed
-
- Finne P, Fallah M, Hakama M, Ciatto S, Hugosson J, de Koning H, Moss S, Nelen V, Auvinen A. Lead-time in the European Randomised Study of Screening for Prostate Cancer. European Journal of Cancer. 2010;46:3102–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

