Defects of CRB2 cause steroid-resistant nephrotic syndrome
- PMID: 25557779
- PMCID: PMC4289689
- DOI: 10.1016/j.ajhg.2014.11.014
Defects of CRB2 cause steroid-resistant nephrotic syndrome
Abstract
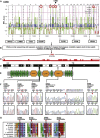
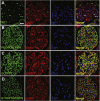
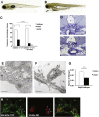
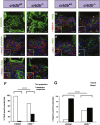
Nephrotic syndrome (NS), the association of gross proteinuria, hypoalbuminaemia, edema, and hyperlipidemia, can be clinically divided into steroid-sensitive (SSNS) and steroid-resistant (SRNS) forms. SRNS regularly progresses to end-stage renal failure. By homozygosity mapping and whole exome sequencing, we here identify recessive mutations in Crumbs homolog 2 (CRB2) in four different families affected by SRNS. Previously, we established a requirement for zebrafish crb2b, a conserved regulator of epithelial polarity, in podocyte morphogenesis. By characterization of a loss-of-function mutation in zebrafish crb2b, we now show that zebrafish crb2b is required for podocyte foot process arborization, slit diaphragm formation, and proper nephrin trafficking. Furthermore, by complementation experiments in zebrafish, we demonstrate that CRB2 mutations result in loss of function and therefore constitute causative mutations leading to NS in humans. These results implicate defects in podocyte apico-basal polarity in the pathogenesis of NS.
Copyright © 2015 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Reiser J., Kriz W., Kretzler M., Mundel P. The glomerular slit diaphragm is a modified adherens junction. J. Am. Soc. Nephrol. 2000;11:1–8. - PubMed
-
- Wiggins R.C. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. - PubMed
-
- Mekahli D., Liutkus A., Ranchin B., Yu A., Bessenay L., Girardin E., Van Damme-Lombaerts R., Palcoux J.B., Cachat F., Lavocat M.P. Long-term outcome of idiopathic steroid-resistant nephrotic syndrome: a multicenter study. Pediatr. Nephrol. 2009;24:1525–1532. - PubMed
-
- ISKDC Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int. 1981;20:765–771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

