Sterically Demanding Unsymmetrical Diaryl-λ(3)-iodanes for Electrophilic Pentafluorophenylation and an Approach to α-Pentafluorophenyl Carbonyl Compounds with an All-Carbon Stereocenter
- PMID: 25558441
- PMCID: PMC4280821
- DOI: 10.1002/open.201402045
Sterically Demanding Unsymmetrical Diaryl-λ(3)-iodanes for Electrophilic Pentafluorophenylation and an Approach to α-Pentafluorophenyl Carbonyl Compounds with an All-Carbon Stereocenter
Erratum in
-
Corrigendum: Sterically Demanding Unsymmetrical Diaryl-λ(3)-iodanes for Electrophilic Pentafluorophenylation and an Approach to α-Pentafluorophenyl Carbonyl Compounds with an All-Carbon Stereocenter.ChemistryOpen. 2016 Jun 1;5(3):267. doi: 10.1002/open.201600044. eCollection 2016 Jun. ChemistryOpen. 2016. PMID: 27551663 Free PMC article.
Abstract
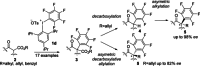
A sterically demanding unsymmetrical pentafluorophenyl-triisopropylphenyl-λ(3)-iodane was developed as an effective reagent for the electrophilic pentafluorophenylation of various β-keto esters and a β-keto amide. 17 examples of α-pentafluorophenylated 1,3-dicarbonyl compounds 3 having a quaternary carbon center are provided. The resulting compounds were nicely transformed into chiral α-pentafluorophenyl ketones with an all-carbon stereogenic center in high yields and high enantioselectivities using asymmetric organocatalysis (up to 98 % ee) or asymmetric metal catalysis (up to 82 % ee).
Keywords: asymmetric organocatalysis; diaryl-λ3-iodanes; electrophilic pentafluorophenylation; palladium.
Figures




Similar articles
-
Corrigendum: Sterically Demanding Unsymmetrical Diaryl-λ(3)-iodanes for Electrophilic Pentafluorophenylation and an Approach to α-Pentafluorophenyl Carbonyl Compounds with an All-Carbon Stereocenter.ChemistryOpen. 2016 Jun 1;5(3):267. doi: 10.1002/open.201600044. eCollection 2016 Jun. ChemistryOpen. 2016. PMID: 27551663 Free PMC article.
-
Chiral N,N'-Dioxide Organocatalyzed Asymmetric Electrophilic α-Cyanation of β-Keto Esters and β-Keto Amides.J Org Chem. 2017 Jan 6;82(1):701-708. doi: 10.1021/acs.joc.6b02726. Epub 2016 Dec 22. J Org Chem. 2017. PMID: 27936694
-
Asymmetric Alkynylation of β-Ketoesters and Naphthols Promoted by New Chiral Biphenylic Iodanes.Chemistry. 2017 Sep 27;23(54):13309-13313. doi: 10.1002/chem.201703238. Epub 2017 Aug 21. Chemistry. 2017. PMID: 28715080
-
Direct Asymmetric Alkylation of Ketones: Still Unconquered.Angew Chem Int Ed Engl. 2017 Aug 1;56(32):9278-9290. doi: 10.1002/anie.201703079. Epub 2017 Jun 27. Angew Chem Int Ed Engl. 2017. PMID: 28497890 Free PMC article. Review.
-
λ3 - and λ5 -Iodanes: Substituent Effects and Pseudorotation/Hypervalent Twisting.Chemistry. 2023 Jun 19;29(34):e202203997. doi: 10.1002/chem.202203997. Epub 2023 May 8. Chemistry. 2023. PMID: 36929780 Review.
Cited by
-
Synthesis of fluoro-functionalized diaryl-λ3-iodonium salts and their cytotoxicity against human lymphoma U937 cells.Beilstein J Org Chem. 2018 Feb 7;14:364-372. doi: 10.3762/bjoc.14.24. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 29507641 Free PMC article.
-
Advances in α-Arylation of Carbonyl Compounds: Diaryliodonium Salts as Arylating Agents.Molecules. 2025 Jul 18;30(14):3019. doi: 10.3390/molecules30143019. Molecules. 2025. PMID: 40733286 Free PMC article. Review.
-
The C3-H Bond Functionalization of Quinoxalin-2(1H)-Ones With Hypervalent Iodine(III) Reagents.Front Chem. 2020 Aug 3;8:582. doi: 10.3389/fchem.2020.00582. eCollection 2020. Front Chem. 2020. PMID: 32850624 Free PMC article. Review.
-
Transition-Metal-Free C-Diarylations to Reach All-Carbon Quaternary Centers.JACS Au. 2024 Aug 5;4(8):2832-2837. doi: 10.1021/jacsau.4c00500. eCollection 2024 Aug 26. JACS Au. 2024. PMID: 39211612 Free PMC article.
-
Iodoarene Activation: Take a Leap Forward toward Green and Sustainable Transformations.Chem Rev. 2025 Mar 26;125(6):3440-3550. doi: 10.1021/acs.chemrev.4c00808. Epub 2025 Mar 7. Chem Rev. 2025. PMID: 40053418 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

