Sterically Demanding Unsymmetrical Diaryl-λ(3)-iodanes for Electrophilic Pentafluorophenylation and an Approach to α-Pentafluorophenyl Carbonyl Compounds with an All-Carbon Stereocenter
- PMID: 25558441
- PMCID: PMC4280821
- DOI: 10.1002/open.201402045
Sterically Demanding Unsymmetrical Diaryl-λ(3)-iodanes for Electrophilic Pentafluorophenylation and an Approach to α-Pentafluorophenyl Carbonyl Compounds with an All-Carbon Stereocenter
Erratum in
-
Corrigendum: Sterically Demanding Unsymmetrical Diaryl-λ(3)-iodanes for Electrophilic Pentafluorophenylation and an Approach to α-Pentafluorophenyl Carbonyl Compounds with an All-Carbon Stereocenter.ChemistryOpen. 2016 Jun 1;5(3):267. doi: 10.1002/open.201600044. eCollection 2016 Jun. ChemistryOpen. 2016. PMID: 27551663 Free PMC article.
Abstract
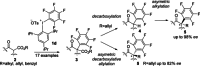
A sterically demanding unsymmetrical pentafluorophenyl-triisopropylphenyl-λ(3)-iodane was developed as an effective reagent for the electrophilic pentafluorophenylation of various β-keto esters and a β-keto amide. 17 examples of α-pentafluorophenylated 1,3-dicarbonyl compounds 3 having a quaternary carbon center are provided. The resulting compounds were nicely transformed into chiral α-pentafluorophenyl ketones with an all-carbon stereogenic center in high yields and high enantioselectivities using asymmetric organocatalysis (up to 98 % ee) or asymmetric metal catalysis (up to 82 % ee).
Keywords: asymmetric organocatalysis; diaryl-λ3-iodanes; electrophilic pentafluorophenylation; palladium.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

