Evaluation of Multi-Atlas Label Fusion for In Vivo MRI Orbital Segmentation
- PMID: 25558466
- PMCID: PMC4280790
- DOI: 10.1117/1.JMI.1.2.024002
Evaluation of Multi-Atlas Label Fusion for In Vivo MRI Orbital Segmentation
Abstract
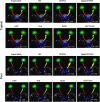
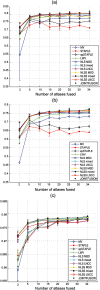
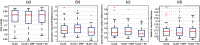
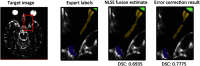
Multi-atlas methods have been successful for brain segmentation, but their application to smaller anatomies remains relatively unexplored. We evaluate 7 statistical and voting-based label fusion algorithms (and 6 additional variants) to segment the optic nerves, eye globes and chiasm. For non-local STAPLE, we evaluate different intensity similarity measures (including mean square difference, locally normalized cross correlation, and a hybrid approach). Each algorithm is evaluated in terms of the Dice overlap and symmetric surface distance metrics. Finally, we evaluate refinement of label fusion results using a learning based correction method for consistent bias correction and Markov random field regularization. The multi-atlas labeling pipelines were evaluated on a cohort of 35 subjects including both healthy controls and patients. Across all three structures, NLSS with a mixed weighting type provided the most consistent results; for the optic nerve NLSS resulted in a median Dice similarity coefficient of 0.81, mean surface distance of 0.41 mm and Hausdorff distance 2.18 mm for the optic nerves. Joint label fusion resulted in slightly superior median performance for the optic nerves (0.82, 0.39 mm and 2.15 mm), but slightly worse on the globes. The fully automated multi-atlas labeling approach provides robust segmentations of orbital structures on MRI even in patients for whom significant atrophy (optic nerve head drusen) or inflammation (multiple sclerosis) is present.
Keywords: Label Fusion; MRI; Multi-Atlas; Optic Nerve; Segmentation.
Figures





Similar articles
-
Robust Optic Nerve Segmentation on Clinically Acquired CT.Proc SPIE Int Soc Opt Eng. 2014 Mar 21;9034:90341G. doi: 10.1117/12.2043715. Proc SPIE Int Soc Opt Eng. 2014. PMID: 24817810 Free PMC article.
-
Comparison of atlas-based techniques for whole-body bone segmentation.Med Image Anal. 2017 Feb;36:98-112. doi: 10.1016/j.media.2016.11.003. Epub 2016 Nov 12. Med Image Anal. 2017. PMID: 27871000
-
Robust Non-Local Multi-Atlas Segmentation of the Optic Nerve.Proc SPIE Int Soc Opt Eng. 2013 Mar 13;8669:86691L. doi: 10.1117/12.2007015. Proc SPIE Int Soc Opt Eng. 2013. PMID: 24478826 Free PMC article.
-
Whole-body bone segmentation from MRI for PET/MRI attenuation correction using shape-based averaging.Med Phys. 2016 Nov;43(11):5848. doi: 10.1118/1.4963809. Med Phys. 2016. PMID: 27806602
-
Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates.Neuroimage. 2014 Nov 1;101:494-512. doi: 10.1016/j.neuroimage.2014.04.054. Epub 2014 Apr 29. Neuroimage. 2014. PMID: 24784800
Cited by
-
Structural-Functional Relationships Between Eye Orbital Imaging Biomarkers and Clinical Visual Assessments.Proc SPIE Int Soc Opt Eng. 2017 Feb 11;10133:101331F. doi: 10.1117/12.2254613. Epub 2017 Feb 24. Proc SPIE Int Soc Opt Eng. 2017. PMID: 28736470 Free PMC article.
-
Automated Segmentation of Tissues Using CT and MRI: A Systematic Review.Acad Radiol. 2019 Dec;26(12):1695-1706. doi: 10.1016/j.acra.2019.07.006. Epub 2019 Aug 10. Acad Radiol. 2019. PMID: 31405724 Free PMC article.
-
Constructing a statistical atlas of the radii of the optic nerve and cerebrospinal fluid sheath in young healthy adults.Proc SPIE Int Soc Opt Eng. 2015 Mar 20;9413:941303. doi: 10.1117/12.2081887. Proc SPIE Int Soc Opt Eng. 2015. PMID: 25914505 Free PMC article.
-
Disambiguating the optic nerve from the surrounding cerebrospinal fluid: Application to MS-related atrophy.Magn Reson Med. 2016 Jan;75(1):414-22. doi: 10.1002/mrm.25613. Epub 2015 Mar 7. Magn Reson Med. 2016. PMID: 25754412 Free PMC article.
-
Multi-Scale Hippocampal Parcellation Improves Atlas-Based Segmentation Accuracy.Proc SPIE Int Soc Opt Eng. 2017 Feb 11;10133:101332D. doi: 10.1117/12.2254425. Epub 2017 Feb 24. Proc SPIE Int Soc Opt Eng. 2017. PMID: 28781411 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

