Kizuna is a novel mitotic substrate for CDC25B phosphatase
- PMID: 25558830
- PMCID: PMC4615109
- DOI: 10.4161/15384101.2014.972882
Kizuna is a novel mitotic substrate for CDC25B phosphatase
Abstract
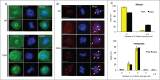
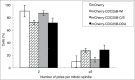
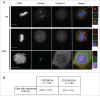
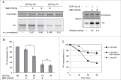
CDC25 dual-specificity phosphatases play a central role in cell cycle control through the activation of Cyclin-Dependent Kinases (CDKs). Expression during mitosis of a stabilized CDC25B mutant (CDC25B-DDA), which cannot interact with the F-box protein βTrCP for proteasome-dependent degradation, causes mitotic defects and chromosome segregation errors in mammalian cells. We found, using the same CDC25B mutant, that stabilization and failure to degrade CDC25B during mitosis lead to the appearance of multipolar spindle cells resulting from a fragmentation of pericentriolar material (PCM) and abolish mitotic Plk1-dependent phosphorylation of Kizuna (Kiz), which is essential for the function of Kiz in maintaining spindle pole integrity. Thus, in mitosis Kiz is a new substrate of CDC25B whose dephosphorylation following CDC25B stabilization leads to the formation of multipolar spindles. Furthermore, endogenous Kiz and CDC25B interact only in mitosis, suggesting that Kiz phosphorylation depends on a balance between CDC25B and Plk1 activities. Our data identify a novel mitotic substrate of CDC25B phosphatase that plays a key role in mitosis control.
Keywords: CDC25B; DMSO, dimethyl-sulfoxyde; DSP, Dithiobis [succinimidyl propionate]; Kiz, Kizuna protein; Kizuna; PCM, pericentriolar material; centrosome; mitosis.
Figures





Similar articles
-
The Plk1 target Kizuna stabilizes mitotic centrosomes to ensure spindle bipolarity.Nat Cell Biol. 2006 Oct;8(10):1095-101. doi: 10.1038/ncb1474. Epub 2006 Sep 17. Nat Cell Biol. 2006. PMID: 16980960
-
Sealed with a Kiz: How Plk1 ensures spindle pole integrity.Dev Cell. 2006 Oct;11(4):431-2. doi: 10.1016/j.devcel.2006.09.012. Dev Cell. 2006. PMID: 17011480
-
Study of the docking-dependent PLK1 phosphorylation of the CDC25B phosphatase.Biochem Biophys Res Commun. 2011 Jun 24;410(1):87-90. doi: 10.1016/j.bbrc.2011.05.110. Epub 2011 May 27. Biochem Biophys Res Commun. 2011. PMID: 21640712
-
Mitotic phosphatases: no longer silent partners.Curr Opin Cell Biol. 2006 Dec;18(6):623-31. doi: 10.1016/j.ceb.2006.09.001. Epub 2006 Oct 9. Curr Opin Cell Biol. 2006. PMID: 17030123 Review.
-
Proteasome-dependent degradation of human CDC25B phosphatase.Mol Biol Rep. 1999 Apr;26(1-2):53-7. doi: 10.1023/a:1006912105352. Mol Biol Rep. 1999. PMID: 10363647 Review.
Cited by
-
The stability of Fbw7α in M-phase requires its phosphorylation by PKC.PLoS One. 2017 Aug 29;12(8):e0183500. doi: 10.1371/journal.pone.0183500. eCollection 2017. PLoS One. 2017. PMID: 28850619 Free PMC article.
-
Two cancer cell lines utilize Myosin 10 and the kinesin HSET differentially to maintain mitotic spindle bipolarity.PLoS One. 2025 May 29;20(5):e0325016. doi: 10.1371/journal.pone.0325016. eCollection 2025. PLoS One. 2025. PMID: 40440343 Free PMC article.
-
Neurogenic decisions require a cell cycle independent function of the CDC25B phosphatase.Elife. 2018 Jul 3;7:e32937. doi: 10.7554/eLife.32937. Elife. 2018. PMID: 29969095 Free PMC article.
-
Phospho-regulation of mitotic spindle assembly.Cytoskeleton (Hoboken). 2020 Dec;77(12):558-578. doi: 10.1002/cm.21649. Epub 2020 Dec 16. Cytoskeleton (Hoboken). 2020. PMID: 33280275 Free PMC article. Review.
-
The BET inhibitor sensitivity is associated with the expression level of CDC25B in pancreatic cancer models.Cancer Drug Resist. 2024 Oct 18;7:40. doi: 10.20517/cdr.2024.53. eCollection 2024. Cancer Drug Resist. 2024. PMID: 39534870 Free PMC article.
References
-
- Morgan DO. Principles of CDK regulation. Nature 1995; 374:131-4; PMID:7877684; http://dx.doi.org/10.1038/374131a0 - DOI - PubMed
-
- Boutros R, Lobjois V, Ducommun B. CDC25 phosphatases in cancer cells: key players? Good targets? Nat Rev Cancer 2007; 7:495-507; PMID:17568790; http://dx.doi.org/10.1038/nrc2169 - DOI - PubMed
-
- Boutros R, Dozier C, Ducommun B. The when and wheres of CDC25 phosphatases. Curr Opin Cell Biol 2006; 18:185-91; PMID:16488126; http://dx.doi.org/10.1016/j.ceb.2006.02.003 - DOI - PubMed
-
- Ferguson AM, White LS, Donovan PJ, Piwnica-Worms H. Normal cell cycle and checkpoint responses in mice and cells lacking Cdc25B and Cdc25C protein phosphatases. Mol Cell Biol 2005; 25:2853-60; PMID:15767688; http://dx.doi.org/10.1128/MCB.25.7.2853-2860.2005 - DOI - PMC - PubMed
-
- Lee G, White LS, Hurov KE, Stappenbeck TS, Piwnica-Worms H. Response of small intestinal epithelial cells to acute disruption of cell division through CDC25 deletion. Proc Natl Acad Sci U S A 2009; 106:4701-6; PMID:19273838; http://dx.doi.org/10.1073/pnas.0900751106 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
