Complexity of naturally produced polybrominated diphenyl ethers revealed via mass spectrometry
- PMID: 25559102
- PMCID: PMC4358748
- DOI: 10.1021/es505440j
Complexity of naturally produced polybrominated diphenyl ethers revealed via mass spectrometry
Abstract
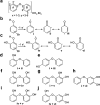
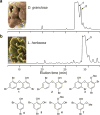
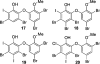
Polybrominated diphenyl ethers (PBDEs) are persistent and bioaccumulative anthropogenic and natural chemicals that are broadly distributed in the marine environment. PBDEs are potentially toxic due to inhibition of various mammalian signaling pathways and enzymatic reactions. PBDE isoforms vary in toxicity in accordance with structural differences, primarily in the number and pattern of hydroxyl moieties afforded upon a conserved core structure. Over four decades of isolation and discovery-based efforts have established an impressive repertoire of natural PBDEs. Based on our recent reports describing the bacterial biosyntheses of PBDEs, we predicted the presence of additional classes of PBDEs to those previously identified from marine sources. Using mass spectrometry and NMR spectroscopy, we now establish the existence of new structural classes of PBDEs in marine sponges. Our findings expand the chemical space explored by naturally produced PBDEs, which may inform future environmental toxicology studies. Furthermore, we provide evidence for iodinated PBDEs and direct attention toward the contribution of promiscuous halogenating enzymes in further expanding the diversity of these polyhalogenated marine natural products.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Global occurrence of polybrominated diphenyl ethers and their hydroxylated and methoxylated structural analogues in an important animal feed (fishmeal).Environ Pollut. 2018 Mar;234:620-629. doi: 10.1016/j.envpol.2017.11.059. Epub 2017 Dec 21. Environ Pollut. 2018. PMID: 29223819
-
Metagenomic discovery of polybrominated diphenyl ether biosynthesis by marine sponges.Nat Chem Biol. 2017 May;13(5):537-543. doi: 10.1038/nchembio.2330. Epub 2017 Mar 20. Nat Chem Biol. 2017. PMID: 28319100 Free PMC article.
-
An assessment of sources and pathways of human exposure to polybrominated diphenyl ethers in the United States.Chemosphere. 2009 Jul;76(4):542-8. doi: 10.1016/j.chemosphere.2009.02.068. Epub 2009 Apr 5. Chemosphere. 2009. PMID: 19349061
-
Polybrominated diphenyl ethers in articles: a review of its applications and legislation.Environ Sci Pollut Res Int. 2017 Feb;24(5):4312-4321. doi: 10.1007/s11356-015-4515-6. Epub 2015 May 20. Environ Sci Pollut Res Int. 2017. PMID: 25987476 Review.
-
Polybrominated diphenyl ether flame retardants in the U.S. marine environment: a review.Environ Int. 2009 Apr;35(3):655-66. doi: 10.1016/j.envint.2008.11.001. Epub 2008 Dec 18. Environ Int. 2009. PMID: 19100622 Review.
Cited by
-
Comprehensive Screening Links Halogenated Organic Compounds with Testosterone Levels in Male Delphinus delphis from the Southern California Bight.Environ Sci Technol. 2018 Mar 6;52(5):3101-3109. doi: 10.1021/acs.est.7b04652. Epub 2018 Feb 15. Environ Sci Technol. 2018. PMID: 29397698 Free PMC article.
-
Polybrominated Diphenyl Ethers: Structure Determination and Trends in Antibacterial Activity.J Nat Prod. 2016 Jul 22;79(7):1872-6. doi: 10.1021/acs.jnatprod.6b00229. Epub 2016 Jul 11. J Nat Prod. 2016. PMID: 27399938 Free PMC article.
-
Sulfonation and glucuronidation of hydroxylated bromodiphenyl ethers in human liver.Chemosphere. 2019 Jul;226:132-139. doi: 10.1016/j.chemosphere.2019.03.103. Epub 2019 Mar 20. Chemosphere. 2019. PMID: 30925405 Free PMC article.
-
40 Years of Research on Polybrominated Diphenyl Ethers (PBDEs)-A Historical Overview and Newest Data of a Promising Anticancer Drug.Molecules. 2021 Feb 13;26(4):995. doi: 10.3390/molecules26040995. Molecules. 2021. PMID: 33668501 Free PMC article. Review.
-
Multi-Omic Profiling of Melophlus Sponges Reveals Diverse Metabolomic and Microbiome Architectures that Are Non-overlapping with Ecological Neighbors.Mar Drugs. 2020 Feb 19;18(2):124. doi: 10.3390/md18020124. Mar Drugs. 2020. PMID: 32092934 Free PMC article.
References
-
- Wiseman SB, Wan Y, Chang H, Zhang X, Hecker M, Jones PD, Giesy JP. Polybrominated diphenyl ethers and their hydroxylated/methoxylated analogs: Environmental sources, metabolic relationships, and relative toxicities. Mar Pollut Bull. 2011;63(5–12):179–88. - PubMed
-
- Segraves EN, Shah RR, Segraves NL, Johnson TA, Whitman S, Sui JK, Kenyon VA, Cichewicz RH, Crews P, Holman TR. Probing the activity differences of simple and complex brominated aryl compounds against 15-soybean, 15-human, and 12-human lipoxygenase. J Med Chem. 2004;47(16):4060–5. - PubMed
-
- Canton RF, Scholten DE, Marsh G, de Jong PC, van den Berg M. Inhibition of human placental aromatase activity by hydroxylated polybrominated diphenyl ethers (OH-PBDEs) Toxicol Appl Pharmacol. 2008;227(1):68–75. - PubMed
-
- de la Fuente JA, Manzanaro S, Martin MJ, de Quesada TG, Reymundo I, Luengo SM, Gago F. Synthesis, activity, and molecular modeling studies of novel human aldose reductase inhibitors based on a marine natural product. J Med Chem. 2003;46(24):5208–21. - PubMed
-
- Legradi J, Dahlberg AK, Cenijn P, Marsh G, Asplund L, Bergman A, Legler J. Disruption of oxidative phosphorylation (OXPHOS) by hydroxylated polybrominated diphenyl ethers (OH-PBDEs) present in the marine environment. Environ Sci Technol. 2014;48(24):14703–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

