Notch ligand Delta-like 1 promotes in vivo vasculogenesis in human cord blood-derived endothelial colony forming cells
- PMID: 25559145
- PMCID: PMC4915927
- DOI: 10.1016/j.jcyt.2014.12.003
Notch ligand Delta-like 1 promotes in vivo vasculogenesis in human cord blood-derived endothelial colony forming cells
Abstract
Background aims: Human cord blood (CB) is enriched in circulating endothelial colony forming cells (ECFCs) that display high proliferative potential and in vivo vessel forming ability. Because Notch signaling is critical for embryonic blood vessel formation in utero, we hypothesized that Notch pathway activation may enhance cultured ECFC vasculogenic properties in vivo.
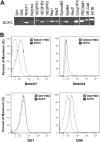
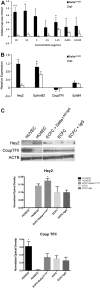
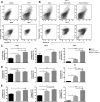
Methods: In vitro ECFC stimulation with an immobilized chimeric Notch ligand (Delta-like1(ext-IgG)) led to significant increases in the mRNA and protein levels of Notch regulated Hey2 and EphrinB2 that were blocked by treatment with γ-secretase inhibitor addition. However, Notch stimulated preconditioning in vitro failed to enhance ECFC vasculogenesis in vivo. In contrast, in vivo co-implantation of ECFCs with OP9-Delta-like 1 stromal cells that constitutively expressed the Notch ligand delta-like 1 resulted in enhanced Notch activated ECFC-derived increased vessel density and enlarged vessel area in vivo, an effect not induced by OP9 control stromal implantation.
Results: This Notch activation was associated with diminished apoptosis in the exposed ECFC.
Conclusions: We conclude that Notch pathway activation in ECFC in vivo via co-implanted stromal cells expressing delta-like 1 promotes vasculogenesis and augments blood vessel formation via diminishing apoptosis of the implanted ECFC.
Keywords: Notch ligand delta-like 1 (Dll1); OP9-Delta-like 1 stromal cells (OP9-DL1); apoptosis; endothelial colony forming cells (ECFCs); vasculogenesis.
Copyright © 2015 International Society for Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


Similar articles
-
Human platelet lysate improves human cord blood derived ECFC survival and vasculogenesis in three dimensional (3D) collagen matrices.Microvasc Res. 2015 Sep;101:72-81. doi: 10.1016/j.mvr.2015.06.006. Epub 2015 Jun 27. Microvasc Res. 2015. PMID: 26122935 Free PMC article.
-
Purification and Functional Characterization of CD34-Expressing Cell Subsets Following Ex Vivo Expansion of Umbilical Cord Blood-Derived Endothelial Colony-Forming Cells.Stem Cells Dev. 2020 Jul;29(14):895-910. doi: 10.1089/scd.2020.0008. Epub 2020 Jun 11. Stem Cells Dev. 2020. PMID: 32336222
-
In vitro effects of stromal cells expressing different levels of Jagged-1 and Delta-1 on the growth of primitive and intermediate CD34(+) cell subsets from human cord blood.Blood Cells Mol Dis. 2011 Dec 15;47(4):205-13. doi: 10.1016/j.bcmd.2011.08.003. Epub 2011 Sep 10. Blood Cells Mol Dis. 2011. PMID: 21911304
-
Ligand-dependent Notch signaling in vascular formation.Adv Exp Med Biol. 2012;727:210-22. doi: 10.1007/978-1-4614-0899-4_16. Adv Exp Med Biol. 2012. PMID: 22399350 Review.
-
Immune-privileged cord blood-derived endothelial colony-forming cells: advancing immunomodulation and vascular regeneration.Angiogenesis. 2025 Mar 6;28(2):19. doi: 10.1007/s10456-025-09973-9. Angiogenesis. 2025. PMID: 40047974 Free PMC article. Review.
Cited by
-
Cystatin C Expression is Promoted by VEGFA Blocking, With Inhibitory Effects on Endothelial Cell Angiogenic Functions Including Proliferation, Migration, and Chorioallantoic Membrane Angiogenesis.J Am Heart Assoc. 2018 Nov 6;7(21):e009167. doi: 10.1161/JAHA.118.009167. J Am Heart Assoc. 2018. PMID: 30571388 Free PMC article.
-
Reduced proliferation of endothelial colony-forming cells in unprovoked venous thromboembolic disease as a consequence of endothelial dysfunction.PLoS One. 2017 Sep 14;12(9):e0183827. doi: 10.1371/journal.pone.0183827. eCollection 2017. PLoS One. 2017. PMID: 28910333 Free PMC article.
-
Epigenetic Activation of Pro-angiogenic Signaling Pathways in Human Endothelial Progenitors Increases Vasculogenesis.Stem Cell Reports. 2017 Nov 14;9(5):1573-1587. doi: 10.1016/j.stemcr.2017.09.009. Epub 2017 Oct 12. Stem Cell Reports. 2017. PMID: 29033304 Free PMC article.
-
Engineering tissue morphogenesis: taking it up a Notch.Trends Biotechnol. 2022 Aug;40(8):945-957. doi: 10.1016/j.tibtech.2022.01.007. Epub 2022 Feb 15. Trends Biotechnol. 2022. PMID: 35181146 Free PMC article. Review.
-
The State of Art of Regenerative Therapy in Cardiovascular Ischemic Disease: Biology, Signaling Pathways, and Epigenetics of Endothelial Progenitor Cells.Cells. 2020 Aug 11;9(8):1886. doi: 10.3390/cells9081886. Cells. 2020. PMID: 32796767 Free PMC article. Review.
References
-
- Niessen K, Karsan A. Notch signaling in cardiac development. Circ Res. 2008;102:1169–81. - PubMed
-
- Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010;32:14–27. - PubMed
-
- Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

