Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability
- PMID: 25559257
- PMCID: PMC4297515
- DOI: 10.1038/ni.3077
Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability
Abstract
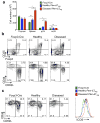
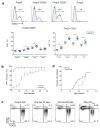
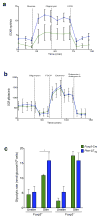
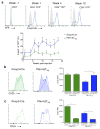
Foxp3(+) regulatory T cells (Treg cells) are required for immunological homeostasis. One notable distinction between conventional T cells (Tconv cells) and Treg cells is differences in the activity of phosphatidylinositol-3-OH kinase (PI(3)K); only Tconv cells downregulate PTEN, the main negative regulator of PI(3)K, upon activation. Here we found that control of PI(3)K in Treg cells was essential for lineage homeostasis and stability. Mice lacking Pten in Treg cells developed an autoimmune-lymphoproliferative disease characterized by excessive T helper type 1 (TH1) responses and B cell activation. Diminished control of PI(3)K activity in Treg cells led to reduced expression of the interleukin-2 (IL-2) receptor α subunit CD25, accumulation of Foxp3(+)CD25(-) cells and, ultimately, loss of expression of the transcription factor Foxp3 in these cells. Collectively, our data demonstrate that control of PI(3)K signaling by PTEN in Treg cells is critical for maintaining their homeostasis, function and stability.
Conflict of interest statement
The authors declare a competing financial interest. For information see,
Figures





Comment in
-
Regulatory T cells. The PTEN stabilizer.Nat Rev Immunol. 2015 Feb;15(2):71. doi: 10.1038/nri3809. Epub 2015 Jan 19. Nat Rev Immunol. 2015. PMID: 25598533
References
-
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. - PubMed
-
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature immunology. 2003;4:330–336. - PubMed
-
- Huynh A, Zhang R, Turka LA. Signals and pathways controlling regulatory T cells. Immunol Rev. 2014;258:117–131. - PubMed
-
- Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI083304/AI/NIAID NIH HHS/United States
- P30 DK063720/DK/NIDDK NIH HHS/United States
- T32 AI007334/AI/NIAID NIH HHS/United States
- R01 HL108006/HL/NHLBI NIH HHS/United States
- T32 HL007627/HL/NHLBI NIH HHS/United States
- P01AI56299/AI/NIAID NIH HHS/United States
- R01 DK105550/DK/NIDDK NIH HHS/United States
- R21 AI105607/AI/NIAID NIH HHS/United States
- 1S10RR023440/RR/NCRR NIH HHS/United States
- P01 HL018646/HL/NHLBI NIH HHS/United States
- T32 AI007529/AI/NIAID NIH HHS/United States
- T32AI007529/AI/NIAID NIH HHS/United States
- R21AI105607/AI/NIAID NIH HHS/United States
- S10 RR023440/RR/NCRR NIH HHS/United States
- R01 AI046643/AI/NIAID NIH HHS/United States
- R56AI083304/AI/NIAID NIH HHS/United States
- 01HL018646/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

