Design, synthesis, and biological evaluation of tetrazole analogs of Cl-amidine as protein arginine deiminase inhibitors
- PMID: 25559347
- PMCID: PMC4610306
- DOI: 10.1021/jm501636x
Design, synthesis, and biological evaluation of tetrazole analogs of Cl-amidine as protein arginine deiminase inhibitors
Abstract
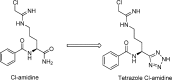
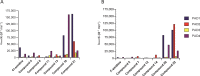
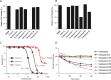
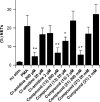
Protein arginine deiminases (PADs) catalyze the post-translational hydrolysis of arginine residues to form citrulline. This once obscure modification is now known to play a key role in the etiology of multiple autoimmune diseases (e.g., rheumatoid arthritis, multiple sclerosis, lupus, and ulcerative colitis) and in some forms of cancer. Among the five human PADs (PAD1, -2, -3, -4, and -6), it is unclear which isozyme contributes to disease pathogenesis. Toward the identification of potent, selective, and bioavailable PAD inhibitors that can be used to elucidate the specific roles of each isozyme, we describe tetrazole analogs as suitable backbone amide bond bioisosteres for the parent pan PAD inhibitor Cl-amidine. These tetrazole based analogs are highly potent and show selectivity toward particular isozymes. Importantly, one of the compounds, biphenyl tetrazole tert-butyl Cl-amidine (compound 13), exhibits enhanced cell killing in a PAD4 expressing osteosarcoma bone marrow (U2OS) cell line and can also block the formation of neutrophil extracellular traps. These bioisosteres represent an important step in our efforts to develop stable, bioavailable, and selective inhibitors for the PADs.
Conflict of interest statement
The authors declare the following competing financial interest(s): P.R.T is a cofounder and consultant to Padlock Therapeutics.
Figures
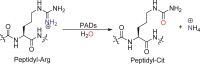
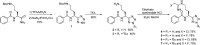
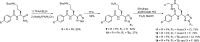

References
-
- Stone E. M.; Schaller T. H.; Bianchi H.; Person M. D.; Fast W. Inactivation of two diverse enzymes in the amidinotransferase superfamily by 2-chloroacetamidine: dimethylargininase and peptidylarginine deiminase. Biochemistry 2005, 44, 13744–13752. - PubMed
-
- Vossenaar E. R.; Zendman A. J.; van Venrooij W. J.; Pruijn G. J. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. BioEssays 2003, 25, 1106–1118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

