Thyrotropin and obesity: increased adipose triglyceride content through glycerol-3-phosphate acyltransferase 3
- PMID: 25559747
- PMCID: PMC4284501
- DOI: 10.1038/srep07633
Thyrotropin and obesity: increased adipose triglyceride content through glycerol-3-phosphate acyltransferase 3
Abstract
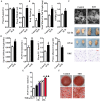
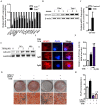
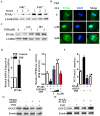
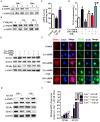
Epidemiological evidence indicates that thyrotropin (TSH) is positively correlated with the severity of obesity. However, the mechanism remains unclear. Here, we show that TSH promoted triglyceride (TG) synthesis in differentiated adipocytes in a thyroid hormone-independent manner. Mice with subclinical hypothyroidism, which is characterized by elevated serum TSH but not thyroid hormone levels, demonstrated a 35% increase in the total white adipose mass compared with their wild-type littermates. Interestingly, Tshr KO mice, which had normal thyroid hormone levels after thyroid hormone supplementation, resisted high-fat diet-induced obesity. TSH could directly induce the activity of glycerol-3-phosphate-acyltransferase 3 (GPAT3), the rate-limiting enzyme in TG synthesis, in differentiated 3T3-L1 adipocytes. However, following either the knockdown of Tshr and PPARγ or the constitutive activation of AMPK, the changes to TSH-triggered GPAT3 activity and adipogenesis disappeared. The over-expression of PPARγ or the expression of an AMPK dominant negative mutant reversed the TSH-induced changes. Thus, TSH acted as a previously unrecognized master regulator of adipogenesis, indicating that modification of the AMPK/PPARγ/GPAT3 axis via the TSH receptor might serve as a potential therapeutic target for obesity.
Figures

References
-
- Steinberger J., Moorehead C., Katch V. & Rocchini A. P. Relationship between insulin resistance and abnormal lipid profile in obese adolescents. J Pediatr 126, 690–695 (1995). - PubMed
-
- Russo P., Lauria F. & Siani A. Heritability of body weight: moving beyond genetics. Nutr Metab Cardiovasc Dis 20, 691–697 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

