Bone marrow minimal residual disease was an early response marker and a consistent independent predictor of survival after anti-GD2 immunotherapy
- PMID: 25559819
- PMCID: PMC4334779
- DOI: 10.1200/JCO.2014.57.6777
Bone marrow minimal residual disease was an early response marker and a consistent independent predictor of survival after anti-GD2 immunotherapy
Abstract
Purpose: Immunotherapy is a standard of care for children with high-risk neuroblastoma, where bone marrow (BM) is the predominant metastatic site. Early response markers of minimal residual disease (MRD) in the BM that are also predictive of survival could help individualize patient therapies.
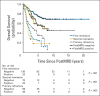
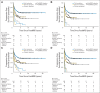
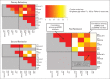
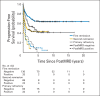
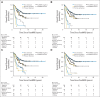
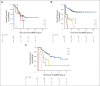
Patients and methods: After achieving first remission (n = 163), primary refractory disease (n = 102), or second remission (n = 95), children with stage 4 neuroblastoma received anti-GD2 3F8 antibody immunotherapy. BM MRD before 3F8 treatment and after cycle 2 (postMRD) was measured using a four-marker panel (B4GALNT1, PHOX2B, CCND1, and ISL1) by quantitative reverse transcription polymerase chain reaction. Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan-Meier method. Prognostic variables were tested in both univariable and multivariable analyses, and MRD markers were further assessed individually and in combination as binary composite (postMRD: 0 and 1) and as equal sum (postMRDSum: 0 to 4) using the Cox regression models, and their predictive accuracy was determined by the concordance index.
Results: When BM was evaluated after cycle 2, individual markers were highly predictive of PFS and OS. The prediction accuracy improved when they were combined in postMRDSum. A multivariable model taking into account all the variables significant in the univariable analyses identified postMRDSum to be independently predictive of PFS and OS. When the model for OS also included missing killer immunoglobulin-like receptor ligand, human antimouse antibody response, and the enrollment disease status, the concordance index was 0.704.
Conclusion: BM MRD after two cycles of immunotherapy was confirmed as an early response marker and a consistent independent predictor of survival.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

References
-
- Cheung NV, Heller G. Chemotherapy dose intensity correlates strongly with response, median survival, and median progression-free survival in metastatic neuroblastoma. J Clin Oncol. 1991;9:1050–1058. - PubMed
-
- Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid: Children's Cancer Group. N Engl J Med. 1999;341:1165–1173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

