Transdermal patches: history, development and pharmacology
- PMID: 25560046
- PMCID: PMC4403087
- DOI: 10.1111/bph.13059
Transdermal patches: history, development and pharmacology
Abstract
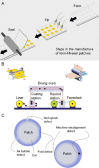
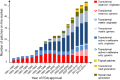
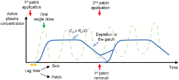
Transdermal patches are now widely used as cosmetic, topical and transdermal delivery systems. These patches represent a key outcome from the growth in skin science, technology and expertise developed through trial and error, clinical observation and evidence-based studies that date back to the first existing human records. This review begins with the earliest topical therapies and traces topical delivery to the present-day transdermal patches, describing along the way the initial trials, devices and drug delivery systems that underpin current transdermal patches and their actives. This is followed by consideration of the evolution in the various patch designs and their limitations as well as requirements for actives to be used for transdermal delivery. The properties of and issues associated with the use of currently marketed products, such as variability, safety and regulatory aspects, are then described. The review concludes by examining future prospects for transdermal patches and drug delivery systems, such as the combination of active delivery systems with patches, minimally invasive microneedle patches and cutaneous solutions, including metered-dose systems.
© 2015 The British Pharmacological Society.
Figures


References
-
- Acrux Ltd. 2014. Data estimated from the ‘US testosterone therapy market histogram’. Annual Report 2014 [Online]. Available at: http://www.acrux.com.au/IRM/Company/ShowPage.aspx/PDFs/1381-10000000/201... (accessed 10/28/2014)
-
- Ahmed SR, Boucher AE, Manni A, Santen RJ, Bartholomew M. Transdermal testosterone therapy in the treatment of male hypogonadism. J Clin Endocrinol Metab. 1988;66:546–551. - PubMed
-
- Aiache JM. Historique des emplâtres. Bull Tech Gattefossé. 1984;77:9–17.
-
- Ale I, Lachapelle J-M, Maibach HI. Skin tolerability associated with transdermal drug delivery systems: an overview. Adv Ther. 2009;26:920–935. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

