Risk factors associated with iatrogenic opioid and benzodiazepine withdrawal in critically ill pediatric patients: a systematic review and conceptual model
- PMID: 25560429
- PMCID: PMC5304939
- DOI: 10.1097/PCC.0000000000000306
Risk factors associated with iatrogenic opioid and benzodiazepine withdrawal in critically ill pediatric patients: a systematic review and conceptual model
Abstract
Objectives: Analgesia and sedation are common therapies in pediatric critical care, and rapid titration of these medications is associated with iatrogenic withdrawal syndrome. We performed a systematic review of the literature to identify all common and salient risk factors associated with iatrogenic withdrawal syndrome and build a conceptual model of iatrogenic withdrawal syndrome risk in critically ill pediatric patients.
Data sources: Multiple databases, including PubMed/Medline, EMBASE, CINAHL, and the Cochrane Central Registry of Clinical Trials, were searched using relevant terms from January 1, 1980, to August 1, 2014.
Study selection: Articles were included if they were published in English and discussed iatrogenic withdrawal syndrome following either opioid or benzodiazepine therapy in children in acute or intensive care settings. Articles were excluded if subjects were neonates born to opioid- or benzodiazepine-dependent mothers, children diagnosed as substance abusers, or subjects with cancer-related pain; if data about opioid or benzodiazepine treatment were not specified; or if primary data were not reported.
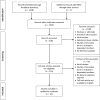
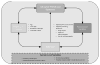
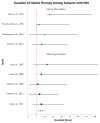
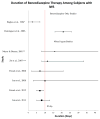
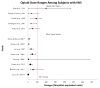
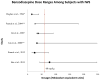
Data extraction: In total, 1,395 articles were evaluated, 33 of which met the inclusion criteria. To facilitate analysis, all opioid and/or benzodiazepine doses were converted to morphine or midazolam equivalents, respectively. A table of evidence was developed for qualitative analysis of common themes, providing a framework for the construction of a conceptual model. The strongest risk factors associated with iatrogenic withdrawal syndrome include duration of therapy and cumulative dose. Additionally, evidence exists linking patient, process, and system factors in the development of iatrogenic withdrawal syndrome.
Findings: Most articles were prospective observational or interventional studies.
Conclusions: Given the state of existing evidence, well-designed prospective studies are required to better characterize iatrogenic withdrawal syndrome in critically ill pediatric patients. This review provides data to support the construction of a conceptual model of iatrogenic withdrawal syndrome risk that, if supported, could be useful in guiding future research.
Figures

Comment in
-
Opioid and benzodiazepine withdrawal syndrome: can we predict and prevent it?Pediatr Crit Care Med. 2015 Feb;16(2):195-6. doi: 10.1097/PCC.0000000000000327. Pediatr Crit Care Med. 2015. PMID: 25647131 No abstract available.
References
-
- Tobias JD. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med. 2000;28(6):2122–32. - PubMed
-
- Franck LS, Vilardi J, Durand D, Powers R. Opioid withdrawal in neonates after continuous infusions of morphine or fentanyl during extracorporeal membrane oxygenation. Am J Crit Care. 1998;7(5):364–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

