CYP17A1 inhibitors in castration-resistant prostate cancer
- PMID: 25560485
- PMCID: PMC4323677
- DOI: 10.1016/j.steroids.2014.12.021
CYP17A1 inhibitors in castration-resistant prostate cancer
Abstract
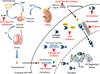
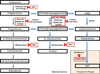
The majority of prostate cancer (PCa) cases are diagnosed as a localized disease. Definitive treatment, active surveillance or watchful waiting are employed as therapeutic paradigms. The current standard of care for the treatment of metastatic PCa is either medical or surgical castration. Once PCa progresses in spite of castrate androgen levels it is termed 'castration-resistant prostate cancer' (CRPC). Patients may even exhibit rising PSA levels with possible bone, lymph node or solid organ metastases. In 2010, the only agent approved for the treatment of CRPC was docetaxel, a chemotherapeutic agent. It is now known that cells from patients with CRPC express androgen receptors (AR) and remain continuously influenced by androgens. As such, treatments with novel hormonal agents that specifically target the biochemical conversion of cholesterol to testosterone have come to the forefront. The use of cytochrome P450c17 (CYP17A1) inhibitor underlies one of the most recent advances in the treatment of CRPC. Abiraterone acetate (AA) was the first CYP17A1 inhibitor approved in the United States. This review will discuss CRPC in general with a specific focus on AA and novel CYP17A1 inhibitors. AA clinical trials will be reviewed along with other novel adjunct treatments that may enhance the effectiveness of abiraterone therapy. Furthermore, the most recently identified CYP17A1 inhibitors Orteronel, Galeterone, VT-464, and CFG920 will also be explored.
Keywords: Abiraterone; Castration resistance; Prostate cancer.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
References
-
- Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. The Journal of urology. 2002;168:9–12. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. - PubMed
-
- Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. The Journal of urology. 2007;177:2106–2131. - PubMed
-
- Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben-Josef E, Mendelson DS, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:1596–1605. - PubMed
-
- Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. The New England journal of medicine. 1998;339:1036–1042. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

