Valganciclovir for the prevention of complications of late cytomegalovirus infection after allogeneic hematopoietic cell transplantation: a randomized trial
- PMID: 25560711
- PMCID: PMC4465336
- DOI: 10.7326/M13-2729
Valganciclovir for the prevention of complications of late cytomegalovirus infection after allogeneic hematopoietic cell transplantation: a randomized trial
Abstract
Background: Optimal prevention of late cytomegalovirus (CMV) disease is poorly defined.
Objective: To compare valganciclovir prophylaxis with polymerase chain reaction-guided preemptive therapy.
Design: Randomized, double-blind trial. (ClinicalTrials.gov: NCT00016068).
Setting: Multicenter trial.
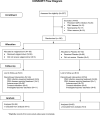
Patients: 184 recipients of hematopoietic cell transplantation (HCT) who were at high risk for late CMV disease (95 patients received valganciclovir and 89 received placebo).
Intervention: 6 months of valganciclovir (900 mg/d) or placebo. Patients with polymerase chain reaction positivity at 1000 copies/mL or greater or a 5-fold increase over baseline were treated with ganciclovir or valganciclovir (5 mg/kg or 900 mg twice daily, respectively).
Measurements: The composite primary end point was death, CMV disease, or other invasive infections by 270 days after HCT. Secondary end points were CMV disease, CMV DNAemia, death, other infections, resource utilization, ganciclovir resistance, quality of life, immune reconstitution, and safety.
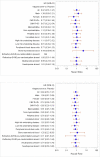
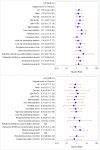
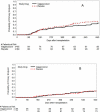
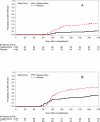
Results: The primary composite outcome occurred in 20% of valganciclovir recipients versus 21% of placebo-preemptive therapy recipients (treatment difference, -0.01 [95% CI, -0.13 to 0.10]; P = 0.86). There was no difference in the primary end point or its components 640 days after HCT. The incidence of a CMV DNAemia level of 1000 copies/mL or greater or a 5-fold increase over baseline was reduced in the valganciclovir group (11% vs. 36%; P < 0.001). Neutropenia was not significantly different at the absolute neutrophil count of less than 0.5 × 109 cells/L (P = 0.57); however, more patients received hematopoietic growth factors in the valganciclovir group (25.3% vs. 12.4%; P = 0.026). No significant differences were seen in other secondary outcomes.
Limitation: Some high-risk patients were not included.
Conclusion: Valganciclovir prophylaxis was not superior in reducing the composite end point of CMV disease, invasive bacterial or fungal disease, or death when compared with polymerase chain reaction-guided preemptive therapy. Both strategies performed similarly with regard to most clinical outcomes.
Primary funding source: Roche Laboratories.
Figures



References
-
- Boeckh M, Gooley TA, Myerson D, Cunningham T, Schoch G, Bowden RA. Cytomegalovirus pp65 antigenemia-guided early treatment with ganciclovir versus ganciclovir at engraftment after allogeneic marrow transplantation: a randomized double-blind study. Blood. 1996;88(10):4063–71. - PubMed
-
- Einsele H, Ehninger G, Hebart H, Wittkowski KM, Schuler U, Jahn G, et al. Polymerase chain reaction monitoring reduces the incidence of cytomegalovirus disease and the duration and side effects of antiviral therapy after bone marrow transplantation. Blood. 1995;86(7):2815–20. - PubMed
-
- Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang M, Myerson D, et al. Late Cytomegalovirus Disease and Mortality in Allogeneic Hematopoietic Stem Cell Transplant Recipients: Importance of Viral Load and T Cell Immunity. Blood. 2003;101:407–14. - PubMed
-
- Krause H, Hebart H, Jahn G, Muller CA, Einsele H. Screening for CMV-specific T cell proliferation to identify patients at risk of developing late onset CMV disease. Bone Marrow Transplantation. 1997;19:1111–6. - PubMed
-
- Nguyen Q, Champlin R, Giralt S, Rolston H, Raad I, Jacobson K, et al. Late cytomegalovirus pneumonia in adult allogeneic blood and marrow transplant recipients. Clinical Infectious Diseases. 1999;28(3):618–23. - PubMed
