Network of GRAS transcription factors involved in the control of arbuscule development in Lotus japonicus
- PMID: 25560877
- PMCID: PMC4348782
- DOI: 10.1104/pp.114.255430
Network of GRAS transcription factors involved in the control of arbuscule development in Lotus japonicus
Abstract
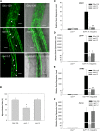
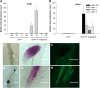
Arbuscular mycorrhizal (AM) fungi, in symbiosis with plants, facilitate acquisition of nutrients from the soil to their host. After penetration, intracellular hyphae form fine-branched structures in cortical cells termed arbuscules, representing the major site where bidirectional nutrient exchange takes place between the host plant and fungus. Transcriptional mechanisms underlying this cellular reprogramming are still poorly understood. GRAS proteins are an important family of transcriptional regulators in plants, named after the first three members: GIBBERELLIC ACID-INSENSITIVE, REPRESSOR of GAI, and SCARECROW. Here, we show that among 45 transcription factors up-regulated in mycorrhizal roots of the legume Lotus japonicus, expression of a unique GRAS protein particularly increases in arbuscule-containing cells under low phosphate conditions and displays a phylogenetic pattern characteristic of symbiotic genes. Allelic rad1 mutants display a strongly reduced number of arbuscules, which undergo accelerated degeneration. In further studies, two RAD1-interacting proteins were identified. One of them is the closest homolog of Medicago truncatula, REDUCED ARBUSCULAR MYCORRHIZATION1 (RAM1), which was reported to regulate a glycerol-3-phosphate acyl transferase that promotes cutin biosynthesis to enhance hyphopodia formation. As in M. truncatula, the L. japonicus ram1 mutant lines show compromised AM colonization and stunted arbuscules. Our findings provide, to our knowledge, new insight into the transcriptional program underlying the host's response to AM colonization and propose a function of GRAS transcription factors including RAD1 and RAM1 during arbuscule development.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures






References
-
- Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435: 824–827 - PubMed
-
- Ané JM, Kiss GB, Riely BK, Penmetsa RV, Oldroyd GE, Ayax C, Lévy J, Debellé F, Baek JM, Kalo P, et al. (2004) Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303: 1364–1367 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

