CXCL10 induces the recruitment of monocyte-derived macrophages into kidney, which aggravate puromycin aminonucleoside nephrosis
- PMID: 25561167
- PMCID: PMC4408165
- DOI: 10.1111/cei.12579
CXCL10 induces the recruitment of monocyte-derived macrophages into kidney, which aggravate puromycin aminonucleoside nephrosis
Abstract
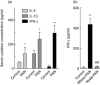
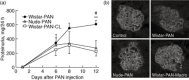
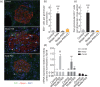
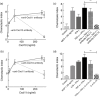
The mechanism responsible for trafficking of monocyte-derived macrophages into kidney in the puromycin aminonucleoside model of nephrotic syndrome in rats (PAN-NS), and the significance of this infiltration, remain largely unknown. CXCL10, a chemokine secreted in many T helper type 1 (Th1) inflammatory diseases, exhibits important roles in trafficking of monocytes and activated T cells. We hypothesized that induction of circulating interferon (IFN)-γ and glomerular tumour necrosis factor (TNF)-α during PAN-NS would stimulate the release of CXCL10 by podocytes, leading to infiltration of activated immune cells and greater glomerular injury. We found that serum IFN-γ, glomerular Cxcl10 mRNA and intra- and peri-glomerular macrophage infiltration were induced strongly during the late acute phase of PAN-NS in Wistar rats, but not in nude (Foxn1(rnu/rnu) ) rats lacking functional effector T lymphocytes. Wistar rats also developed significantly greater proteinuria than nude rats, which could be abolished by macrophage depletion. Stimulation of cultured podocytes with both IFN-γ and TNF-α markedly induced the expression of Cxcl10 mRNA and CXCL10 secretion. Together, these data support our hypothesis that increased circulating IFN-γ and glomerular TNF-α induce synergistically the production and secretion of CXCL10 by podocytes, attracting activated macrophages into kidney tissue. The study also suggests that IFN-γ, secreted from Th1 lymphocytes, may prime proinflammatory macrophages that consequently aggravate renal injury.
Keywords: CXCL10; chemokines; kidney injury; macrophages; nephrotic syndrome.
© 2015 British Society for Immunology.
Figures

References
-
- Schreiner GF, Cotran RS, Unanue ER. Modulation of Ia and leukocyte common antigen expression in rat glomeruli during the course of glomerulonephritis and aminonucleoside nephrosis. Lab Invest. 1984;51:524–533. - PubMed
-
- Diamond JR, Pesek I, Ruggieri S, Karnovsky MJ. Essential fatty acid deficiency during acute puromycin nephrosis ameliorates late renal injury. Am J Physiol. 1989;257:F798–807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

