Tet1-mediated DNA demethylation regulates neuronal cell death induced by oxidative stress
- PMID: 25561289
- PMCID: PMC4284502
- DOI: 10.1038/srep07645
Tet1-mediated DNA demethylation regulates neuronal cell death induced by oxidative stress
Erratum in
-
ERRATUM: Tet1-mediated DNA demethylation regulates neuronal cell death induced by oxidative stress.Sci Rep. 2015 Mar 12;5:8951. doi: 10.1038/srep08951. Sci Rep. 2015. PMID: 25762513 Free PMC article. No abstract available.
Abstract
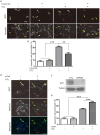
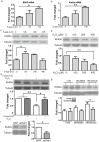
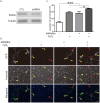
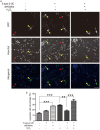
Epigenetic regulations including DNA methylation and demethylation play critical roles in neural development. However, whether DNA methylation and demethylation may play a role in neuronal cell death remains largely unclear. Here we report that the blockade of DNA methyltransferase inhibits apoptosis of cerebellar granule cells and cortical neurons in response to oxidative stress. We found that knockdown of ten-eleven translocation methylcytosine dioxygenase (Tet1), a critical enzyme for DNA demethylation, significantly increase apoptosis of cerebellar granule cells induced by hydrogen peroxide. Moreover, cerebellar granule cells from tet1(null) mice appeared to be more sensitive to oxidative stress, suggesting the critical role of Tet1 in neuronal cell death. We further showed that the expression of Klotho, an antiaging protein, in cerebellar granule cells is tightly regulated by DNA methylation. Finally, we found that knockdown of Klotho diminished the rescue effects of DNA methyltransferase inhibitors and Tet1 on neuronal cell death induced by oxidative stress. Our work revealed the role of Tet1-mediated DNA demethylation on neuronal protection against oxidative stress and provided the molecular mechanisms underlying the epigenetic regulation of neuronal cell death, suggesting the role of Klotho in regulating neuronal cell death in response to oxidative stress.
Figures

Similar articles
-
Tet1 Overexpression and Decreased DNA Hydroxymethylation Protect Neurons Against Cell Death After Injury by Increasing Expression of Genes Involved in Cell Survival.World Neurosurg. 2019 Jun;126:e713-e722. doi: 10.1016/j.wneu.2019.02.133. Epub 2019 Mar 5. World Neurosurg. 2019. PMID: 30849555
-
Non-catalytic roles for TET1 protein negatively regulating neuronal differentiation through srGAP3 in neuroblastoma cells.Protein Cell. 2016 May;7(5):351-61. doi: 10.1007/s13238-016-0267-4. Epub 2016 Apr 25. Protein Cell. 2016. PMID: 27113584 Free PMC article.
-
Tet1 overexpression leads to anxiety-like behavior and enhanced fear memories via the activation of calcium-dependent cascade through Egr1 expression in mice.FASEB J. 2018 Jan;32(1):390-403. doi: 10.1096/fj.201601340RR. Epub 2017 Sep 12. FASEB J. 2018. PMID: 28899881
-
Multiple Functions of Ten-eleven Translocation 1 during Tumorigenesis.Chin Med J (Engl). 2016 Jul 20;129(14):1744-51. doi: 10.4103/0366-6999.185873. Chin Med J (Engl). 2016. PMID: 27411465 Free PMC article. Review.
-
Advances in the DNA methylation hydroxylase TET1.Biomark Res. 2021 Oct 16;9(1):76. doi: 10.1186/s40364-021-00331-7. Biomark Res. 2021. PMID: 34656178 Free PMC article. Review.
Cited by
-
Assessment and site-specific manipulation of DNA (hydroxy-)methylation during mouse corticogenesis.Life Sci Alliance. 2019 Feb 27;2(2):e201900331. doi: 10.26508/lsa.201900331. Print 2019 Apr. Life Sci Alliance. 2019. PMID: 30814272 Free PMC article.
-
Novel Treatment Strategies for the Nervous System: Circadian Clock Genes, Non-coding RNAs, and Forkhead Transcription Factors.Curr Neurovasc Res. 2018;15(1):81-91. doi: 10.2174/1567202615666180319151244. Curr Neurovasc Res. 2018. PMID: 29557749 Free PMC article. Review.
-
Klotho gene silencing promotes pathology in the mdx mouse model of Duchenne muscular dystrophy.Hum Mol Genet. 2016 Jun 15;25(12):2465-2482. doi: 10.1093/hmg/ddw111. Epub 2016 May 6. Hum Mol Genet. 2016. PMID: 27154199 Free PMC article.
-
Antioxidant and Antidiabetic Activity of Algae.Life (Basel). 2023 Feb 7;13(2):460. doi: 10.3390/life13020460. Life (Basel). 2023. PMID: 36836817 Free PMC article. Review.
-
α-Klotho Expression in Mouse Tissues Following Acute Exhaustive Exercise.Front Physiol. 2019 Dec 10;10:1498. doi: 10.3389/fphys.2019.01498. eCollection 2019. Front Physiol. 2019. PMID: 31920703 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

