Animal models to assess the abuse liability of tobacco products: effects of smokeless tobacco extracts on intracranial self-stimulation
- PMID: 25561387
- PMCID: PMC4337227
- DOI: 10.1016/j.drugalcdep.2014.12.015
Animal models to assess the abuse liability of tobacco products: effects of smokeless tobacco extracts on intracranial self-stimulation
Abstract
Background: Preclinical models are needed to inform regulation of tobacco products by the Food and Drug Administration (FDA). Typically, animal models of tobacco addiction involve exposure to nicotine alone or nicotine combined with isolated tobacco constituents (e.g. minor alkaloids). The goal of this study was to develop a model using extracts derived from tobacco products that contain a range of tobacco constituents to more closely model product exposure in humans.
Methods: This study compared the addiction-related effects of nicotine alone and nicotine dose-equivalent concentrations of aqueous smokeless tobacco extracts on intracranial self-stimulation (ICSS) in rats. Extracts were prepared from Kodiak Wintergreen, a conventional product, or Camel Snus, a potential "modified risk tobacco product". Binding affinities of nicotine alone and extracts at various nicotinic acetylcholine receptor (nAChR) subtypes were also compared.
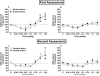
Results: Kodiak and Camel Snus extracts contained levels of minor alkaloids within the range of those shown to enhance nicotine's behavioral effects when studied in isolation. Nonetheless, acute injection of both extracts produced reinforcement-enhancing (ICSS threshold-decreasing) effects similar to those of nicotine alone at low to moderate nicotine doses, as well as similar reinforcement-attenuating/aversive (ICSS threshold-increasing) effects at high nicotine doses. Extracts and nicotine alone also had similar binding affinity at all nAChRs studied.
Conclusions: Relative nicotine content is the primary pharmacological determinant of the abuse liability of Kodiak and Camel Snus as measured using ICSS. These models may be useful to compare the relative abuse liability of other tobacco products and to model FDA-mandated changes in product performance standards.
Keywords: Extract; Intracranial self-stimulation; Nicotine; Non-nicotine tobacco constituents; Policy; Smokeless tobacco.
Copyright © 2015. Published by Elsevier Ireland Ltd.
Figures
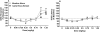
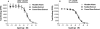
Similar articles
-
Propylene glycol, a major electronic cigarette constituent, attenuates the adverse effects of high-dose nicotine as measured by intracranial self-stimulation in rats.Drug Alcohol Depend. 2018 Dec 1;193:162-168. doi: 10.1016/j.drugalcdep.2018.08.037. Epub 2018 Oct 18. Drug Alcohol Depend. 2018. PMID: 30384324 Free PMC article.
-
Delivery of nicotine in an extract of a smokeless tobacco product reduces its reinforcement-attenuating and discriminative stimulus effects in rats.Psychopharmacology (Berl). 2012 Apr;220(3):565-76. doi: 10.1007/s00213-011-2514-y. Epub 2011 Sep 30. Psychopharmacology (Berl). 2012. PMID: 21960181 Free PMC article.
-
Effects of nicotine and minor tobacco alkaloids on intracranial-self-stimulation in rats.Drug Alcohol Depend. 2015 Aug 1;153:330-4. doi: 10.1016/j.drugalcdep.2015.06.005. Epub 2015 Jun 9. Drug Alcohol Depend. 2015. PMID: 26094184 Free PMC article.
-
Consortium on Methods Evaluating Tobacco: Research Tools to Inform US Food and Drug Administration Regulation of Snus.Nicotine Tob Res. 2018 Sep 25;20(11):1292-1300. doi: 10.1093/ntr/ntx228. Nicotine Tob Res. 2018. PMID: 29059363 Free PMC article. Review.
-
Smokeless tobacco: an addicting drug.Adv Dent Res. 1997 Sep;11(3):330-5. doi: 10.1177/08959374970110030401. Adv Dent Res. 1997. PMID: 9524433 Review.
Cited by
-
Status and Future Directions of Preclinical Behavioral Pharmacology in Tobacco Regulatory Science.Behav Anal (Wash D C). 2018 Aug;18(3):252-274. doi: 10.1037/bar0000113. Epub 2018 Jul 9. Behav Anal (Wash D C). 2018. PMID: 30214916 Free PMC article.
-
Evaluation of Sex Differences in the Elasticity of Demand for Nicotine and Food in Rats.Nicotine Tob Res. 2020 May 26;22(6):925-934. doi: 10.1093/ntr/ntz171. Nicotine Tob Res. 2020. PMID: 31603225 Free PMC article.
-
Smokeless tobacco extract inhibits proliferation and promotes apoptosis in oral mucous fibroblasts.Oncol Lett. 2018 Oct;16(4):5066-5074. doi: 10.3892/ol.2018.9252. Epub 2018 Aug 2. Oncol Lett. 2018. PMID: 30250574 Free PMC article.
-
Propylene glycol, a major electronic cigarette constituent, attenuates the adverse effects of high-dose nicotine as measured by intracranial self-stimulation in rats.Drug Alcohol Depend. 2018 Dec 1;193:162-168. doi: 10.1016/j.drugalcdep.2018.08.037. Epub 2018 Oct 18. Drug Alcohol Depend. 2018. PMID: 30384324 Free PMC article.
-
Comprehensive Chemical Characterization of Natural American Spirit Cigarettes.Tob Regul Sci. 2019 Jul;5(4):381-399. doi: 10.18001/trs.5.4.8. Tob Regul Sci. 2019. PMID: 33907702 Free PMC article.
References
-
- Ambrose V, Miller JH, Dickson SJ, Hampton S, Truman P, Lea RA, Fowles J. Tobacco particulate matter is more potent than nicotine at upregulating nicotinic receptors on SH-SY5Y cells. Nicotine Tob. Res. 2007;9:793–799. - PubMed
-
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology. 1999;146:290–296. - PubMed
-
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci. Biobehav. Rev. 1995;19:39–51. - PubMed
-
- Bauco P, Wise RA. Potentiation of lateral hypothalamic and midline mesencephalic brain stimulation reinforcement by nicotine: examination of repeated treatment. J. Pharmacol. Exp. Ther. 1994;271:294–301. - PubMed
-
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

