Stably paused genes revealed through inhibition of transcription initiation by the TFIIH inhibitor triptolide
- PMID: 25561494
- PMCID: PMC4281563
- DOI: 10.1101/gad.246173.114
Stably paused genes revealed through inhibition of transcription initiation by the TFIIH inhibitor triptolide
Abstract
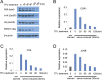
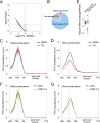
Transcription by RNA polymerase II (Pol II) in metazoans is regulated in several steps, including preinitiation complex (PIC) formation, initiation, Pol II escape, productive elongation, cotranscriptional RNA processing, and termination. Genome-wide studies have demonstrated that the phenomenon of promoter-bound Pol II pausing is widespread, especially for genes involved in developmental and stimulus-responsive pathways. However, a mechanistic understanding of the paused Pol II state at promoters is limited. For example, at a global level, it is unclear to what extent the engaged paused Pol II is stably tethered to the promoter or undergoes rapid cycles of initiation and termination. Here we used the small molecule triptolide (TPL), an XPB/TFIIH inhibitor, to block transcriptional initiation and then measured Pol II occupancy by chromatin immunoprecipitation (ChIP) followed by next-generation sequencing (ChIP-seq). This inhibition of initiation enabled us to investigate different states of paused Pol II. Specifically, our global analysis revealed that most genes with paused Pol II, as defined by a pausing index, show significant clearance of Pol II during the period of TPL treatment. Our study further identified a group of genes with unexpectedly stably paused Pol II, with unchanged Pol II occupancy even after 1 h of inhibition of initiation. This group of genes constitutes a small portion of all paused genes defined by the conventional criterion of pausing index. These findings could pave the way for evaluating the contribution of different elongation/pausing factors on different states of Pol II pausing in developmental and other stimulus-responsive pathways.
Keywords: RNA polymerase II; chromatin; transcription.
© 2015 Chen et al.; Published by Cold Spring Harbor Laboratory Press.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
