Role of positron emission tomography for the monitoring of response to therapy in breast cancer
- PMID: 25561512
- PMCID: PMC4319634
- DOI: 10.1634/theoncologist.2014-0342
Role of positron emission tomography for the monitoring of response to therapy in breast cancer
Abstract
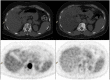
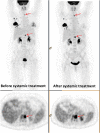
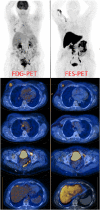
This review considers the potential utility of positron emission tomography (PET) tracers in the setting of response monitoring in breast cancer, with a special emphasis on glucose metabolic changes assessed with (18)F-fluorodeoxyglucose (FDG). In the neoadjuvant setting of breast cancer, the metabolic response can predict the final complete pathologic response after the first cycles of chemotherapy. Because tumor metabolic behavior highly depends on cancer subtype, studies are ongoing to define the optimal metabolic criteria of tumor response in each subtype. The recent multicentric randomized AVATAXHER trial has suggested, in the human epidermal growth factor 2-positive subtype, a clinical benefit of early tailoring the neoadjuvant treatment in women with poor metabolic response after the first course of treatment. In the bone-dominant metastatic setting, there is increasing clinical evidence that FDG-PET/computed tomography (CT) is the most accurate imaging modality for assessment of the tumor response to treatment when both metabolic information and morphologic information are considered. Nevertheless, there is a need to define standardized metabolic criteria of response, including the heterogeneity of response among metastases, and to evaluate the costs and health outcome of FDG-PET/CT compared with conventional imaging. New non-FDG radiotracers highlighting specific molecular hallmarks of breast cancer cells have recently emerged in preclinical and clinical studies. These biomarkers can take into account the heterogeneity of tumor biology in metastatic lesions. They may provide valuable clinical information for physicians to select and monitor the effectiveness of novel therapeutics targeting the same molecular pathways of breast tumor.
Keywords: 18F-Fluorodeoxyglucose; Breast; Cancer; Monitoring; Positron emission tomography; Response.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
Similar articles
-
18F-FDG PET/CT for early prediction of response to neoadjuvant lapatinib, trastuzumab, and their combination in HER2-positive breast cancer: results from Neo-ALTTO.J Nucl Med. 2013 Nov;54(11):1862-8. doi: 10.2967/jnumed.112.119271. Epub 2013 Oct 3. J Nucl Med. 2013. PMID: 24092940 Clinical Trial.
-
Estrogen receptor-positive/human epidermal growth factor receptor 2-negative breast tumors: early prediction of chemosensitivity with (18)F-fluorodeoxyglucose positron emission tomography/computed tomography during neoadjuvant chemotherapy.Cancer. 2013 Jun 1;119(11):1960-8. doi: 10.1002/cncr.28020. Epub 2013 Mar 15. Cancer. 2013. PMID: 23504954
-
18F-fluorodeoxyglucose (FDG) PET/CT after two cycles of neoadjuvant therapy may predict response in HER2-negative, but not in HER2-positive breast cancer.Oncotarget. 2015 Oct 6;6(30):29388-95. doi: 10.18632/oncotarget.5001. Oncotarget. 2015. PMID: 26336821 Free PMC article.
-
Response to therapy in breast cancer.J Nucl Med. 2009 May;50 Suppl 1:55S-63S. doi: 10.2967/jnumed.108.057240. Epub 2009 Apr 20. J Nucl Med. 2009. PMID: 19380410 Review.
-
¹⁸F-FDG PET/CT for Monitoring of Treatment Response in Breast Cancer.J Nucl Med. 2016 Feb;57 Suppl 1(Suppl 1):34S-9S. doi: 10.2967/jnumed.115.157875. J Nucl Med. 2016. PMID: 26834099 Free PMC article. Review.
Cited by
-
Complete Metabolic Response on Interim 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography to Predict Long-Term Survival in Patients with Breast Cancer Undergoing Neoadjuvant Chemotherapy.Oncologist. 2017 May;22(5):526-534. doi: 10.1634/theoncologist.2016-0334. Epub 2017 Apr 4. Oncologist. 2017. PMID: 28377466 Free PMC article.
-
Impact of Using Uniform Attenuation Coefficients for Heterogeneously Dense Breasts in a Dedicated Breast PET/X-ray Scanner.IEEE Trans Radiat Plasma Med Sci. 2020 Sep;4(5):585-593. doi: 10.1109/trpms.2020.2991120. Epub 2020 Apr 29. IEEE Trans Radiat Plasma Med Sci. 2020. PMID: 33163753 Free PMC article.
-
The Role of (18)F-FDG PET/CT and MRI in Assessing Pathological Complete Response to Neoadjuvant Chemotherapy in Patients with Breast Cancer: A Systematic Review and Meta-Analysis.Biomed Res Int. 2016;2016:3746232. doi: 10.1155/2016/3746232. Epub 2016 Feb 15. Biomed Res Int. 2016. PMID: 26981529 Free PMC article.
-
[Advances on correlation of PET-CT findings with breast cancer molecular subtypes, treatment response and prognosis].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2017 May 25;46(5):473-480. doi: 10.3785/j.issn.1008-9292.2017.10.04. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2017. PMID: 29488712 Free PMC article. Chinese.
-
Systemic treatment in breast cancer: a primer for radiologists.Insights Imaging. 2016 Feb;7(1):131-44. doi: 10.1007/s13244-015-0447-4. Epub 2015 Nov 13. Insights Imaging. 2016. PMID: 26567115 Free PMC article.
References
-
- Groheux D, Espié M, Giacchetti S, et al. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266:388–405. - PubMed
-
- Wahl RL, Zasadny K, Helvie M, et al. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: Initial evaluation. J Clin Oncol. 1993;11:2101–2111. - PubMed
-
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. - PubMed
-
- Gralow JR, Burstein HJ, Wood W, et al. Preoperative therapy in invasive breast cancer: Pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26:814–819. - PubMed
-
- Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

