Control of stem cell self-renewal and differentiation by the heterochronic genes and the cellular asymmetry machinery in Caenorhabditis elegans
- PMID: 25561544
- PMCID: PMC4311799
- DOI: 10.1073/pnas.1422852112
Control of stem cell self-renewal and differentiation by the heterochronic genes and the cellular asymmetry machinery in Caenorhabditis elegans
Abstract
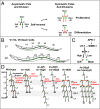
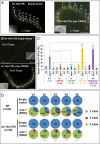
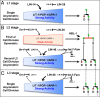
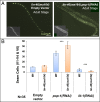
Transitions between asymmetric (self-renewing) and symmetric (proliferative) cell divisions are robustly regulated in the context of normal development and tissue homeostasis. To genetically assess the regulation of these transitions, we used the postembryonic epithelial stem (seam) cell lineages of Caenorhabditis elegans. In these lineages, the timing of these transitions is regulated by the evolutionarily conserved heterochronic pathway, whereas cell division asymmetry is conferred by a pathway consisting of Wnt (Wingless) pathway components, including posterior pharynx defect (POP-1)/TCF, APC related/adenomatosis polyposis coli (APR-1)/APC, and LIT-1/NLK (loss of intestine/Nemo-like kinase). Here we explore the genetic regulatory mechanisms underlying stage-specific transitions between self-renewing and proliferative behavior in the seam cell lineages. We show that mutations of genes in the heterochronic developmental timing pathway, including lin-14 (lineage defect), lin-28, lin-46, and the lin-4 and let-7 (lethal defects)-family microRNAs, affect the activity of LIT-1/POP-1 cellular asymmetry machinery and APR-1 polarity during larval development. Surprisingly, heterochronic mutations that enhance LIT-1 activity in seam cells can simultaneously also enhance the opposing, POP-1 activity, suggesting a role in modulating the potency of the cellular polarizing activity of the LIT-1/POP-1 system as development proceeds. These findings illuminate how the evolutionarily conserved cellular asymmetry machinery can be coupled to microRNA-regulated developmental pathways for robust regulation of stem cell maintenance and proliferation during the course of development. Such genetic interactions between developmental timing regulators and cell polarity regulators could underlie transitions between asymmetric and symmetric stem cell fates in other systems and could be deregulated in the context of developmental disorders and cancer.
Keywords: asymmetric division; heterochronic; lit-1/Nlk; pop-1/Tcf; stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Wnt signaling controls temporal identities of seam cells in Caenorhabditis elegans.Dev Biol. 2010 Sep 15;345(2):144-55. doi: 10.1016/j.ydbio.2010.07.002. Epub 2010 Jul 17. Dev Biol. 2010. PMID: 20624379
-
Role of PRY-1/Axin in heterochronic miRNA-mediated seam cell development.BMC Dev Biol. 2019 Jul 15;19(1):17. doi: 10.1186/s12861-019-0197-5. BMC Dev Biol. 2019. PMID: 31307392 Free PMC article.
-
C. elegans Runx/CBFβ suppresses POP-1 TCF to convert asymmetric to proliferative division of stem cell-like seam cells.Development. 2019 Nov 18;146(22):dev180034. doi: 10.1242/dev.180034. Development. 2019. PMID: 31740621 Free PMC article.
-
An elegant miRror: microRNAs in stem cells, developmental timing and cancer.Chromosoma. 2009 Aug;118(4):405-18. doi: 10.1007/s00412-009-0210-z. Epub 2009 Apr 3. Chromosoma. 2009. PMID: 19340450 Free PMC article. Review.
-
Control of developmental timing by micrornas and their targets.Annu Rev Cell Dev Biol. 2002;18:495-513. doi: 10.1146/annurev.cellbio.18.012502.105832. Epub 2002 Apr 2. Annu Rev Cell Dev Biol. 2002. PMID: 12142272 Review.
Cited by
-
The multifaceted roles of microRNAs in differentiation.Curr Opin Cell Biol. 2020 Dec;67:118-140. doi: 10.1016/j.ceb.2020.08.015. Epub 2020 Nov 3. Curr Opin Cell Biol. 2020. PMID: 33152557 Free PMC article. Review.
-
Comparison of cell cycle components, apoptosis and cytoskeleton-related molecules and therapeutic effects of flavopiridol and geldanamycin on the mouse fibroblast, lung cancer and embryonic stem cells.Tumour Biol. 2016 Sep;37(9):12423-12440. doi: 10.1007/s13277-016-5108-9. Epub 2016 Jun 21. Tumour Biol. 2016. PMID: 27324070
-
HSP-4/BiP expression in secretory cells is regulated by a developmental program and not by the unfolded protein response.PLoS Biol. 2019 Mar 25;17(3):e3000196. doi: 10.1371/journal.pbio.3000196. eCollection 2019 Mar. PLoS Biol. 2019. PMID: 30908491 Free PMC article.
-
Mechanisms of lineage specification in Caenorhabditis elegans.Genetics. 2023 Dec 6;225(4):iyad174. doi: 10.1093/genetics/iyad174. Genetics. 2023. PMID: 37847877 Free PMC article.
-
Asymmetric Wnt Pathway Signaling Facilitates Stem Cell-Like Divisions via the Nonreceptor Tyrosine Kinase FRK-1 in Caenorhabditis elegans.Genetics. 2015 Nov;201(3):1047-60. doi: 10.1534/genetics.115.181412. Epub 2015 Sep 9. Genetics. 2015. PMID: 26358719 Free PMC article.
References
-
- Clevers H. Stem cells, asymmetric division and cancer. Nat Genet. 2005;37(10):1027–1028. - PubMed
-
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441(7097):1068–1074. - PubMed
-
- Grifoni D, et al. aPKCzeta cortical loading is associated with Lgl cytoplasmic release and tumor growth in Drosophila and human epithelia. Oncogene. 2007;26(40):5960–5965. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

