Therapeutic Potential of Adipose-Derived SSEA-3-Positive Muse Cells for Treating Diabetic Skin Ulcers
- PMID: 25561682
- PMCID: PMC4303359
- DOI: 10.5966/sctm.2014-0181
Therapeutic Potential of Adipose-Derived SSEA-3-Positive Muse Cells for Treating Diabetic Skin Ulcers
Abstract
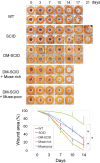
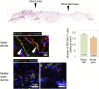
Stage-specific embryonic antigen-3 (SSEA-3)-positive multipotent mesenchymal cells (multilineage differentiating stress-enduring [Muse] cells) were isolated from cultured human adipose tissue-derived stem/stromal cells (hASCs) and characterized, and their therapeutic potential for treating diabetic skin ulcers was evaluated. Cultured hASCs were separated using magnetic-activated cell sorting into positive and negative fractions, a SSEA-3+ cell-enriched fraction (Muse-rich) and the remaining fraction (Muse-poor). Muse-rich hASCs showed upregulated and downregulated pluripotency and cell proliferation genes, respectively, compared with Muse-poor hASCs. These cells also released higher amounts of certain growth factors, particularly under hypoxic conditions, compared with Muse-poor cells. Skin ulcers were generated in severe combined immunodeficiency (SCID) mice with type 1 diabetes, which showed delayed wound healing compared with nondiabetic SCID mice. Treatment with Muse-rich cells significantly accelerated wound healing compared with treatment with Muse-poor cells. Transplanted cells were integrated into the regenerated dermis as vascular endothelial cells and other cells. However, they were not detected in the surrounding intact regions. Thus, the selected population of ASCs has greater therapeutic effects to accelerate impaired wound healing associated with type 1 diabetes. These cells can be achieved in large amounts with minimal morbidity and could be a practical tool for a variety of stem cell-depleted or ischemic conditions of various organs and tissues.
Keywords: Adipose; Adult stem cells; Cell culture; Endothelial cell; Hyaluronan; Mesenchymal stem cells; Stem cell transplantation; Tissue regeneration.
©AlphaMed Press.
Figures



References
-
- Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. - PubMed
-
- Harris RG, Herzog EL, Bruscia EM, et al. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305:90–93. - PubMed
-
- Toma JG, Akhavan M, Fernandes KJ, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

