Glycogen synthase kinase-3 (GSK3) inhibition induces prosurvival autophagic signals in human pancreatic cancer cells
- PMID: 25561726
- PMCID: PMC4342473
- DOI: 10.1074/jbc.M114.616714
Glycogen synthase kinase-3 (GSK3) inhibition induces prosurvival autophagic signals in human pancreatic cancer cells
Abstract
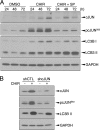
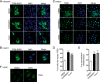
Glycogen synthase kinase-3 (GSK3) are ubiquitously expressed serine-threonine kinases involved in a plethora of functions ranging from the control of glycogen metabolism to transcriptional regulation. We recently demonstrated that GSK3 inhibition triggers JNK-cJUN-dependent apoptosis in human pancreatic cancer cells. However, the comprehensive picture of downstream GSK3-regulated pathways/functions remains elusive. Herein, counterbalancing the death signals, we show that GSK3 inhibition induces prosurvival signals through increased activity of the autophagy/lysosomal network. Our data also reveal a contribution of GSK3 in the regulation of the master transcriptional regulator of autophagy and lysosomal biogenesis, transcription factor EB (TFEB) in pancreatic cancer cells. Similarly to mammalian target of rapamycin (mTOR) inhibition, GSK3 inhibitors promote TFEB nuclear localization and leads to TFEB dephosphorylation through endogenous serine/threonine phosphatase action. However, GSK3 and mTOR inhibition impinge differently and independently on TFEB phosphorylation suggesting that TFEB is regulated by a panel of kinases and/or phosphatases. Despite their differential impact on TFEB phosphorylation, both GSK3 and mTOR inhibitors promote 14-3-3 dissociation and TFEB nuclear localization. Quantitative mass spectrometry analyses further reveal an increased association of TFEB with nuclear proteins upon GSK3 and mTOR inhibition suggesting a positive impact on TFEB transcriptional function. Finally, a predominant nuclear localization of TFEB is unveiled in fully fed pancreatic cancer cells, whereas a reduction in TFEB expression significantly impairs their capacity for growth in an anchorage-independent manner. In addition, TFEB-restricted cells are more sensitive to apoptosis upon GSK3 inhibition. Altogether, our data uncover new functions under the control of GSK3 in pancreatic cancer cells in addition to providing key insight into TFEB regulation.
Keywords: 14-3-3 Protein; Apoptosis; Autophagy; Glycogen Synthase Kinase 3 (GSK-3); Pancreatic Cancer; Transcription Factor.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Bardeesy N., DePinho R. A. (2002) Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2, 897–909 - PubMed
-
- Hezel A. F., Kimmelman A. C., Stanger B. Z., Bardeesy N., Depinho R. A. (2006) Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 20, 1218–1249 - PubMed
-
- Schneider G., Siveke J. T., Eckel F., Schmid R. M. (2005) Pancreatic cancer: basic and clinical aspects. Gastroenterology 128, 1606–1625 - PubMed
-
- Jones S., Zhang X., Parsons D. W., Lin J. C., Leary R. J., Angenendt P., Mankoo P., Carter H., Kamiyama H., Jimeno A., Hong S. M., Fu B., Lin M. T., Calhoun E. S., Kamiyama M., Walter K., Nikolskaya T., Nikolsky Y., Hartigan J., Smith D. R., Hidalgo M., Leach S. D., Klein A. P., Jaffee E. M., Goggins M., Maitra A., Iacobuzio-Donahue C., Eshleman J. R., Kern S. E., Hruban R. H., Karchin R., Papadopoulos N., Parmigiani G., Vogelstein B., Velculescu V. E., Kinzler K. W. (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321, 1801–1806 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

