Biochemical reconstitution and phylogenetic comparison of human SET1 family core complexes involved in histone methylation
- PMID: 25561738
- PMCID: PMC4358272
- DOI: 10.1074/jbc.M114.627646
Biochemical reconstitution and phylogenetic comparison of human SET1 family core complexes involved in histone methylation
Abstract
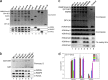
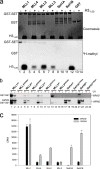
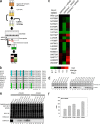
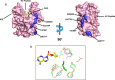
Mixed lineage leukemia protein-1 (MLL1) is a member of the SET1 family of histone H3 lysine 4 (H3K4) methyltransferases that are required for metazoan development. MLL1 is the best characterized human SET1 family member, which includes MLL1-4 and SETd1A/B. MLL1 assembles with WDR5, RBBP5, ASH2L, DPY-30 (WRAD) to form the MLL1 core complex, which is required for H3K4 dimethylation and transcriptional activation. Because all SET1 family proteins interact with WRAD in vivo, it is hypothesized they are regulated by similar mechanisms. However, recent evidence suggests differences among family members that may reflect unique regulatory inputs in the cell. Missing is an understanding of the intrinsic enzymatic activities of different SET1 family complexes under standard conditions. In this investigation, we reconstituted each human SET1 family core complex and compared subunit assembly and enzymatic activities. We found that in the absence of WRAD, all but one SET domain catalyzes at least weak H3K4 monomethylation. In the presence of WRAD, all SET1 family members showed stimulated monomethyltransferase activity but differed in their di- and trimethylation activities. We found that these differences are correlated with evolutionary lineage, suggesting these enzyme complexes have evolved to accomplish unique tasks within metazoan genomes. To understand the structural basis for these differences, we employed a "phylogenetic scanning mutagenesis" assay and identified a cluster of amino acid substitutions that confer a WRAD-dependent gain-of-function dimethylation activity on complexes assembled with the MLL3 or Drosophila trithorax proteins. These results form the basis for understanding how WRAD differentially regulates SET1 family complexes in vivo.
Keywords: Ash2L; Cancer; Epigenetics; Histone Methylation; Leukemia; MLL; Phylogenetics; Product Specificity; SET1; WDR5.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Santos-Rosa H., Schneider R., Bannister A. J., Sherriff J., Bernstein B. E., Emre N. C., Schreiber S. L., Mellor J., Kouzarides T. (2002) Active genes are tri-methylated at K4 of histone H3. Nature 419, 407–411 - PubMed
-
- Pokholok D. K., Harbison C. T., Levine S., Cole M., Hannett N. M., Lee T. I., Bell G. W., Walker K., Rolfe P. A., Herbolsheimer E., Zeitlinger J., Lewitter F., Gifford D. K., Young R. A. (2005) Genome-wide map of nucleosome acetylation and methylation in yeast. Cell 122, 517–527 - PubMed
-
- Heintzman N. D., Stuart R. K., Hon G., Fu Y., Ching C. W., Hawkins R. D., Barrera L. O., Van Calcar S., Qu C., Ching K. A., Wang W., Weng Z., Green R. D., Crawford G. E., Ren B. (2007) Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 39, 311–318 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

