Amantadine as Augmentation in Managing Opioid Withdrawal with Clonidine: a randomized controlled trial
- PMID: 25561954
- PMCID: PMC4277803
Amantadine as Augmentation in Managing Opioid Withdrawal with Clonidine: a randomized controlled trial
Abstract
Objective: Withdrawal symptoms are a main reason of continuous use of opioid. This study compares the efficacy of augmentation of amantadine with clonidine in decreasing opioid withdrawal symptoms.
Methods: This double-blind randomized clinical trial was carried out in the detoxification and rehabilitation inpatient ward at Razi Hospital, Tabriz, Iran during 2012. The patients were randomly assigned to receive clonidine or clonidine plus amantadine; and withdrawal symptoms were evaluated in the admission day and 24, 48, and 72 hours later. Data were analyzed using SPSS by the 2*2 repeated analyses of variances (ANOVA).
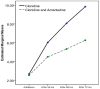
Results: From the total of 69 participants, 30 patients completed the trial in each group. The severity of symptoms, however, had an increasing trend in both groups. Analysis of variance of the symptom severity score (by The Clinical Opiate Withdrawal Scale) revealed a significant group-time interaction, and the patients who were receiving amantadine experienced milder symptoms.
Conclusions: Treatment of opioid withdrawal symptoms with amantadine and clonidine would result in a better outcome compared with clonidine alone.
Keywords: Amantadine; Clonidine; Withdrawal Syndrome.
Figures
Similar articles
-
The therapeutic effect of adding dextromethorphan to clonidine for reducing symptoms of opioid withdrawal: a randomized clinical trial.ISRN Psychiatry. 2013 Jun 20;2013:546030. doi: 10.1155/2013/546030. Print 2013. ISRN Psychiatry. 2013. PMID: 23864983 Free PMC article.
-
Passionflower in the treatment of opiates withdrawal: a double-blind randomized controlled trial.J Clin Pharm Ther. 2001 Oct;26(5):369-73. doi: 10.1046/j.1365-2710.2001.00366.x. J Clin Pharm Ther. 2001. PMID: 11679027 Clinical Trial.
-
Lofexidine versus diazepam for the treatment of opioid withdrawal syndrome: A double-blind randomized clinical trial in Singapore.J Subst Abuse Treat. 2018 Aug;91:1-11. doi: 10.1016/j.jsat.2018.04.012. Epub 2018 Apr 25. J Subst Abuse Treat. 2018. PMID: 29910009 Clinical Trial.
-
Reduction of opiate withdrawal symptoms with use of clonidine in a county jail.J Correct Health Care. 2015 Jan;21(1):27-34. doi: 10.1177/1078345814557630. Epub 2014 Nov 26. J Correct Health Care. 2015. PMID: 25431436 Review.
-
Lofexidine, an {alpha}2-receptor agonist for opioid detoxification.Ann Pharmacother. 2010 Feb;44(2):343-51. doi: 10.1345/aph.1M347. Epub 2009 Dec 29. Ann Pharmacother. 2010. PMID: 20040696 Review.
Cited by
-
Non-Opioid Neurotransmitter Systems that Contribute to the Opioid Withdrawal Syndrome: A Review of Preclinical and Human Evidence.J Pharmacol Exp Ther. 2019 Nov;371(2):422-452. doi: 10.1124/jpet.119.258004. Epub 2019 Aug 7. J Pharmacol Exp Ther. 2019. PMID: 31391211 Free PMC article. Review.
-
Pharmacological therapies for management of opium withdrawal.Cochrane Database Syst Rev. 2018 Jun 21;6(6):CD007522. doi: 10.1002/14651858.CD007522.pub2. Cochrane Database Syst Rev. 2018. PMID: 29929212 Free PMC article.
-
A narrative review of outcome measures used in drug and alcohol inpatient withdrawal treatment research.Drug Alcohol Rev. 2023 Feb;42(2):415-426. doi: 10.1111/dar.13591. Epub 2023 Jan 12. Drug Alcohol Rev. 2023. PMID: 36633552 Free PMC article. Review.
References
-
- Assadi SM, Hafezi M, Mokri A, Razzaghi EM, Ghaeli P. Opioid detoxification using high doses of buprenorphine in 24 hours: a randomized, double blind, controlled clinical trial. J Subst Abuse Treat. 2004;27:75–82. - PubMed
-
- Gold MS, Redmond DE, Jr, Kleber HD. Clonidine blocks acute opiate-withdrawal symptoms. Lancet. 1978;2:599–602. - PubMed
-
- Raith K, Hochhaus G. Drugs used in the treatment of opioid tolerance and physical dependence: a review. Int J Clin Pharmacol Ther. 2004;42:191–203. - PubMed
-
- Bisaga A, Comer SD, Ward AS, Popik P, Kleber HD, Fischman MW. The NMDA antagonist memantine attenuates the expression of opioid physical dependence in humans. Psychopharmacology (Berl) 2001;157:1–10. - PubMed
-
- Sadock BJ, Sadock VA. Kaplan and Sadock’s Comprehensive Textbook of psychiatry. 7. Philadelphia: Lippincot Williams & Wilkins; 2000. pp. 941–1057.
LinkOut - more resources
Full Text Sources