The Analysis of the Relationship between Multiple Myeloma Cells and Their Microenvironment
- PMID: 25561981
- PMCID: PMC4280399
- DOI: 10.7150/jca.10873
The Analysis of the Relationship between Multiple Myeloma Cells and Their Microenvironment
Abstract
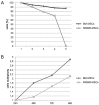
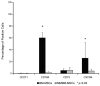
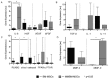
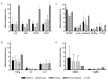
The bone marrow microenvironment plays a key role in the stimulation of growth and survival of multiple myeloma (MM) cells. We investigated whether membrane microfragments (MFBs) exert a stimulatory effect on mesenchymal stem cell (MSC) gene expression or differentiation. MSCs from patients with multiple myeloma (MMBM-MSCs) proliferated at a slower rate than MSCs from healthy volunteers (BM-MSCs), and fewer MMBM-MSCs adhered to the substrate as compared to BM-MSCs. Phenotypic analysis revealed that MMBM-MSCs and BM-MSCs differed significantly in terms of their CD166 and CXCR4 expressions. In conclusion, our comparative analysis of mesenchymal cells from MM patients and healthy volunteers revealed differences in the genetic and phenotypic profiles of these two populations, their potential for osteodifferentiation, and expression of surface antigens. Moreover, we showed that membrane MFBs may alter the genetic profile of MSCs, leading to disorders of their osteodifferentiation, and interact with the WNT pathway via presentation of the DKK-1 protein.
Keywords: bone marrow; genotype; membrane microfragments; microenvironment; multiple myeloma; osteodifferentiation.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Derksen PW, de Gorter DJ, Meijer HP. et al. The hepatocyte growth factor/Met pathway controls proliferation and apoptosis in multiple myeloma. Leukemia. 2003;17:764–74. - PubMed
-
- Gahrton G. New therapeutic targets in multiple myeloma. Lancet. 2004;364:1648–9. - PubMed
-
- Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–74. - PubMed
-
- De Raeve HR, Vanderkerken K. The role of the bone marrow microenvironment in multiple myeloma. Histol Histopathol. 2005;20:1227–50. - PubMed
-
- Podar K, Tai YT, Lin BK. et al. Vascular endothelial growth factor-induced migration of multiple myeloma cells is associated with beta 1 integrin- and phosphatidylinositol 3-kinase-dependent PKC alpha activation. J Biol Chem. 2002;277:7875–81. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

