Ocular pharmacokinetics of fluocinolone acetonide following Iluvien implantation in the vitreous humor of rabbits
- PMID: 25562126
- PMCID: PMC4286586
- DOI: 10.1089/jop.2014.0100
Ocular pharmacokinetics of fluocinolone acetonide following Iluvien implantation in the vitreous humor of rabbits
Abstract
Purpose: The purpose of this study was to evaluate the systemic and ocular pharmacokinetics (PK) of fluocinolone acetonide (FAc) following administration of Iluvien(®) intravitreal implants.
Methods: The FAc intravitreal implant was administered to rabbits in 3 doses (0.2, 0.5, and 1.0 μg/day). The concentration of FAc was measured by a validated liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) method in plasma and ocular tissues at various time points through month 24.
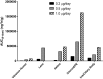
Results: Following administration of the 0.2 μg/day implant, FAc levels peaked in most tissues at day 2 or 8, reached approximate steady state levels by month 3 and very gradually decreased over the duration of the study. The FAc level in the aqueous humor was not measurable at most time points in the rabbit. FAc was still present in most ocular tissues at 2 years. The 0.5 and 1.0 μg/day dose groups followed the same pattern through month 9. The elimination half lives in the tissues for which it was measurable were greater than 83 days. Exposure to FAc was highest in the choroid/retinal pigment epithelium for all doses, followed by lens and retina.
Conclusions: The results of this study demonstrate sustained delivery of FAc from the Iluvien intravitreal implant in the ocular tissue of rabbits. Retina and lens FAc levels with the Iluvien implant were approximately 1/10 those reported with the Retisert(®) implant. FAc levels in the aqueous were not measureable with Iluvien where they were measured for 12 months with Retisert.
Figures

References
-
- Jager R.D., Aiello L.P., Patel S.C., and Cunningham E.T.Risks of intravitreous injection: a comprehensive review. Retina. 24:676–698, 2004 - PubMed
-
- RETISERT® [Package Insert]. Bausch and Lomb, Inc., May2012
-
- ILUVIEN® Summary of Product Characteristics. Alpharetta, GA: Alimera Sciences Limited; December2013
-
- Driot J.-Y., Novack G.D., Rittenhouse K.D., Milazzo C., and Pearson P.A.Ocular pharmacokinetics of fluocinolone acetonide after RETISERT™ Intravitreal Implantation in rabbits over a 1-year period. J. Ocular Pharm. Ther. 20:269–275, 2004 - PubMed
-
- Campochiaro P.A., Nguyen Q.D., Hafiz G., Bloom S., Busquets M., Ciulla T., Feiner L., Sabates N., Billman K., Kapik B., Green K., Kane F.E., and FAMOUS Study Group. Aqueous levels of fluocinolone acetonide after administration of fluocinolone acetonide inserts or fluocinolone acetonide implants. Ophthalmology. 120:583–587, 2013 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
