Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis
- PMID: 25562322
- PMCID: PMC4319435
- DOI: 10.1172/JCI74894
Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis
Erratum in
-
Corrigendum. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis.J Clin Invest. 2015 Mar 2;125(3):1364. doi: 10.1172/JCI81442. Epub 2015 Mar 2. J Clin Invest. 2015. PMID: 25729857 Free PMC article. No abstract available.
Abstract
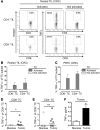
The composition of tumor-targeted T cell infiltrates is a major prognostic factor in colorectal cancer (CRC) outcome; however, the functional role of these populations in prolonging patient survival remains unclear. Here, we evaluated 190 patients with CRC for the presence of functionally active tumor-infiltrating lymphocytes (TILs), the tumor specificity of these TILs, and the correlation between patient TILs and long-term survival. Using intracytoplasmic cytokine staining in conjunction with HLA multimers loaded with tumor peptide and antigen-specific cytokine secretion assays, we determined that TNF-α expression delineates a population of tumor antigen-specific (TA-specific) cytotoxic T lymphocytes (CTLs) present within tumors from patients with CRC. Upregulation of TNF-α expression in TILs strongly correlated with an increase in the total amount of intratumoral TNF-α, which is indicative of tumor-specific CTL activity. Moreover, a retrospective multivariate analysis of 102 patients with CRC, which had multiple immune parameters evaluated, revealed that increased TNF-α concentration was an independent prognostic factor. Together, these results indicate that the prognostic impact of T cell infiltrates for CRC maybe largely based on subpopulations of active TA-specific T cells within the tumor, suggesting causal implication for these cells in patient survival. Additionally, these results support the use of intratumoral TNF-α, which is indicative of T cell function, as a prognostic parameter for CRC.
Figures
References
-
- Funada Y, Noguchi T, Kikuchi R, Takeno S, Uchida Y, Gabbert HE. Prognostic significance of CD8+ T cell and macrophage peritumoral infiltration in colorectal cancer. Oncol Rep. 2003;10(2):309–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

