Cardiac effects of in-utero exposure to antiretroviral therapy in HIV-uninfected children born to HIV-infected mothers
- PMID: 25562493
- PMCID: PMC4287991
- DOI: 10.1097/QAD.0000000000000499
Cardiac effects of in-utero exposure to antiretroviral therapy in HIV-uninfected children born to HIV-infected mothers
Abstract
Objectives: We evaluated the potential cardiac effects of in-utero exposures to antiretroviral drugs in HIV-exposed but uninfected (HEU) children.
Design and methods: We compared echocardiographic parameters of left ventricular function (ejection fraction, fractional shortening, and stress-velocity index) and structure (left ventricular dimension, posterior wall/septal thickness, mass, thickness-to-dimension ratio, and wall stress) (expressed as Z-scores to account for age and body surface area) between HEU and HIV-unexposed cohorts from the Pediatric HIV/AIDS Cohort Study's Surveillance Monitoring for ART Toxicities study. Within the HEU group, we investigated the associations between the echocardiographic Z-scores and in-utero exposures to maternal antiretroviral drugs.
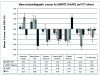
Results: There were no significant differences in echocardiographic Z-scores between 417 HEU and 98 HIV-unexposed children aged 2-7 years. Restricting the analysis to HEU children, first-trimester exposures to combination antiretroviral therapy (a regimen including at least three antiretroviral drugs) and to certain specific antiretroviral drugs were associated with significantly lower stress-velocity Z-scores (mean decreases of 0.22-0.40 SDs). Exposure to combination antiretroviral therapy was also associated with lower left ventricular dimension Z-scores (mean decrease of 0.44 SD). First-trimester exposure to combination antiretroviral therapy was associated with higher mean left ventricular posterior wall thickness and lower mean left ventricular wall stress Z-scores.
Conclusion: There was no evidence of significant cardiac toxicity of perinatal combination antiretroviral therapy exposure in HEU children. Subclinical differences in left ventricular structure and function with specific in-utero antiretroviral exposures indicate the need for a longitudinal cardiac study in HEU children to assess long-term cardiac risk and cardiac monitoring recommendations.
Conflict of interest statement
None of the authors have any conflicts of interest or disclaimers or have received compensation regarding this study.
Figures
References
-
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;33:1173–1180. - PubMed
-
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Diaz C, Hayani K, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–494. - PubMed
-
- Jackson JB, Musoke P, Fleming T, Guay LA, Bagenda D, Allen M, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. - PubMed
-
- Blanche S, Tardieu M, Rustin P, Slama A, Barret B, Firtion G, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–1089. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

